3963
Studying cerebrospinal fluid bulk flow in mice using high-resolution dynamic macromolecular gadolinium enhanced MRI on a whole-body 3T system1Imaging Sciences, University of Rochester Medical Center, Rochester, NY, United States, 2Tufts University, Boston, MA, United States, 3University of Rochester, Rochester, NY, United States
Synopsis
Study of CSF flow can be used to assess CSF clearance pathways for the better understanding of neurological diseases, such as Alzheimer's disease. We developed a technique for macromolecular gadolinium enhanced dynamic MRI of CSF in mice on a whole-body 3T system. A specially designed 4-channel RF receive coil was developed, and a high-resolution 3D spoiled gradient-echo T1 sequence was used. Gadolinium-albumin contrast was injected into the cisterna magna. Contrast enhancement was detected in various locations including the olfactory lobe, nasal cavity, lymphatic system and spine cord. Lower contrast elimination rates were observed in old mice than in young mice.
INTRODUCTION
The study of cerebrospinal fluid (CSF) flow can be used to assess CSF clearance pathways to gain a better understanding of its role in neurological diseases, such as Alzheimer’s disease [1]. CSF flow in small animal models may be studied using MRI [2]. While small-bore animal MRI systems with very high static magnetic field and field gradients are advantageous for small animal MRI, whole-body 3T systems have also be used successfully for imaging small animals such as mice [3]. The goal of this study is to develop a technique for gadolinium (Gd) contrast enhanced dynamic MR imaging of CSF flow in mice on a whole-body 3T system.METHODS
The study was conducted on a Siemens PRISMA Fit 3T system with approval from the Institutional Animal Care and Use Committee. A dedicated 4-channel receive coil with signal coverage from the neck to the nose of adult mice was designed and constructed (Fig. 1). It has a cylindrical shape that tapered anteriorly and conformed to the mouse shape for optimal signal sensitivity. The coil consists of 4 elements arranged along the coil circumference and the coil enclosure was made of 3D printed acrylic material. A detachable cradle piece was used to facilitate the positioning and immobilization of the mouse. Six mice (C57BL/6, three young (3-month) and three old (20-month) were imaged. During MRI, each mouse was placed in the prone position on the cradle, properly aligned and secured in position by taping the head and body to the cradle. The animal was then slid into the coil with the head and neck inside the coil (Fig. 1). The mouse was anesthetized using isoflurane administered through a tube to the anterior opening of the coil, and was kept warm (37oC) by placing a circulating warm water pad (T/Pump Professional, Stryker Medical, Michigan, USA) over the mouse. Bovine serum albumin (BSA) Gd contrast agent (5 μL at 0.5 μL/min) was injected into the cisterna magna at the start of the second scan by a power injector (MRI Compatible Syringe Pump, Harvard Apparatus) placed inside the MRI scan room. A series of 12 continuous MRI measurements were acquired using a 3D spoiled gradient-echo T1-weighted sequence with repetition time = 50 ms, echo time = 6.67 ms, field-of-view = 38mm, acquisition matrix = 128 x 128, slice thickness = 0.3mm, flip angle = 25o, receive bandwidth = 130Hz/pixel, and isotropic resolution of 0.3x0.3x0.3 mm3. The scan time for each measurement was 8:31 mins, resulting in a total scan time of 1 hr 42 mins for the 12 measurements. After scanning, the data were post-processed and analyzed on an iMAC workstation using the Osirix software with the “DCE Tool” plugin. Time plots of the contrast signal enhancement at different regions of interest were generated, and the averaged results of the young and the old mice were compared.RESULTS
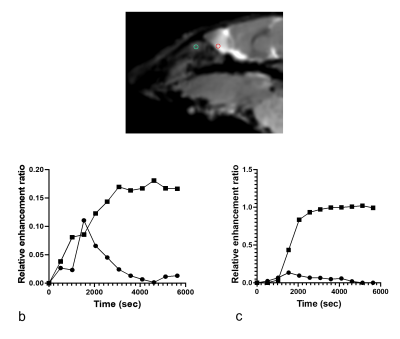
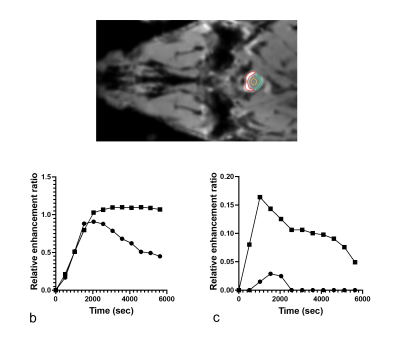
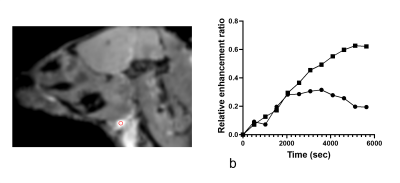
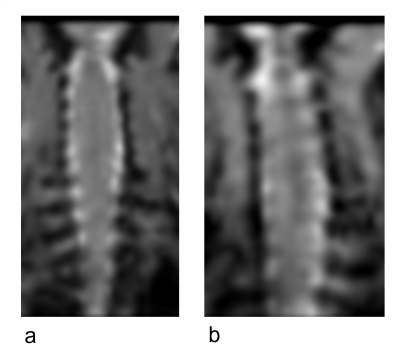
Gadolinium signal enhancement was detected mostly in the ventral spinal subarachnoid space of the brain and spinal cord and also in the lymph nodes (Figs 2-4). The contrast elimination rates were profoundly lower in the old mice than the young mice. In addition, there were CSF dispersal from the spinal nerve roots (Fig 5) and along the Circle of Willis but no significant signal in the spinal canal and brain parenchyma.DISCUSSION
In this study, the specially designed RF receive coil has provided high signal sensitivity needed for mouse imaging at 3T. Although imaging mice is more demanding than imaging larger animals such as rats, mice have the advantage of being easier to be genetically manipulated for diseases.The slow flow rate of CSF allowed the use of much lower temporal resolution than typical dynamic contrast enhanced imaging, and enabled the acquisition of high-resolution 3D data that captured the CSF flow in mice. The resolution is sufficient for most regions of interest except the dura mater. In a recent near-infrared (NIR) study in mice, it was found that the dura mater was not a main CSF flow clearance pathway [4], indicating it might not need to be observed by MRI.
In this study, a macromolecular BSA-Gd contrast agent was used instead of the common small molecular Gd agents to reduce its diffusion into tissues. This approach represents bulk flow of CSF more effectively along the CSF drainage pathways [2]. The slower contrast elimination observed in the old mice in this study suggests that delayed CSF outflow could be associated with the onset of brain aging, neurodegeneration and age-related decline in cognition.
CONCLUSION
Our study has demonstrated the feasibility of conducting high-resolution 3D macromolecular gadolinium enhanced dynamic study of mouse CSF flow on a whole-body 3T scanner. This technique should facilitate a wider use of MRI for the studies of CSF clearance and/or lymphatic clearance in small rodent models of neurological diseases.Acknowledgements
This study is supported by a National Institute of Health grant 1RF1AG057574 and also by a pilot grant from the University of Rochester Center for Advanced Brain Imaging & Neurophysiology.References
1. Chiu C, Miller MC, Caralopoulos IN, et al. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS. 2012;9:3.
2. Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299-309.
3. Herrmann, K.H., Schmidt, S., Kretz, A., Haenold, R., Krumbein, I., Metzler, M., Gaser, C., Witte, O.W., & Reichenbach, J.R. (2012) Possibilities and limitations for high resolution small animal MRI on a clinical whole-body 3T scanner. Magnetic Resonance Materials in Physics, Biology and Medicine, 25, 233–244.
4. Brady M, Rahman A, Combs A, et al. Cerebrospinal fluid drainage kinetics across the cribriform plate are reduced with aging. Fluids Barriers CNS.2020;17:71
Figures