3958
Quantitative T2* and T1 mapping of post-mortem MS tissue at 7T for discrimination of normal-appearing and dirty-appearing white matter pathology1Biological and Biomedical Engineering, McGill University, Montreal, QC, Canada, 2McConnell Brain Imaging Centre, Montreal, QC, Canada, 3Neurology and Neurosurgery, McGill University, Montreal, QC, Canada, 4Montreal Neurological Institute-Hospital, Montreal, QC, Canada
Synopsis
Quantitative T2* and T1 relaxometry metrics calculated at 7T have high sensitivity to myelin and can be jointly used to identify dirty-appearing white matter (DAWM) and normal-appearing white matter (NAWM) pathology in post-mortem, multiple sclerosis (MS) brain tissue. Relaxometry mapping was performed on fixed, cerebral brain samples from MS patients and healthy donors. T2* and T1 distributions in WM from MS tissue exhibited bimodality and were shifted to higher relaxation time values compared to healthy tissue. Using k-means clustering applied to 2D T2* and T1 MS tissue data, regions of DAWM were detected in periventricular WM for MS tissue samples.
Introduction and Motivation
Multiple Sclerosis (MS) is a neurodegenerative disease marked by inflammation, demyelination, neuronal loss and axonal degeneration. The pathological features of MS manifest in white matter (WM) and gray matter (GM) as both focal plaques and diffuse tissue injury, varying with MS phenotype, disease duration and type of disease modifying therapy1.Apparent transverse relaxation time (T2*) and longitudinal relaxation time (T1) are quantitative relaxometry metrics sensitive to myelin and iron in the brain. T2* and T1 can be applied to assess the extent and spatial distribution of diffuse WM pathology in MS2. Histopathological and imaging studies of post-mortem MS brain tissue have revealed two distinct types of diffuse non-plaque WM changes: normal-appearing WM (NAWM) and dirty-appearing WM (DAWM). NAWM is characterized by demyelination, inflammation, axonal loss, and lengthened T2* and T1 times3, 4. In contrast, DAWM shows greater demyelination, greater increase in T2* and T1 times, and typically comprises regions with indefinite boundaries3. However, the optimal quantitative MRI method for unique discrimination of DAWM from NAWM and an understanding of the underlying MS pathological substrates is an ongoing area of investigation.
The motivation for this work was to apply high-resolution, robust 7T quantitative T2* and T1 mapping of post-mortem MS brain tissue to identify and discriminate NAWM and DAWM.
Methods
Brain Samples: Nine post-mortem, human cerebral brain sections were obtained from the Douglas-Bell Canada Brain Bank (duration of formalin fixation ≥ 10 years). Specifically, 6 sections were obtained from two MS patient donors (Patient #1: Frontal, middle and caudal large sections; Patient #2: Frontal small, frontal large and caudal sections) and 3 sections were obtained from two healthy control donors (Control #1: Frontal and caudal sections; Control #2: Caudal section).MR Scanning: Brain sections were scanned using a Siemens 7T Scanner (MAGNETOM Terra) with a 1Tx/32Rx head coil (Nova Medical). Acquisitions included a slab-selective 3D Multi-Echo (ME) GRE sequence (0.32mm3, TR = 46ms, TE = [6.84, 11.57, 16.30, 21.03, 25.76, 30.49]ms, FA = 15o, 25 averages) and a 3D MP2RAGE sequence (0.32x0.32x0.64mm3, TR/TE = 3000/2.08ms, Turbo Factor = 24, echo spacing = 6.6ms, FA1/FA2 = 8/8o, TI1/TI2 = 183/900ms, 25 averages). A 2.5mm3 B1+ map was acquired using Siemens’ Turbo-Flash B1+ mapping protocol.
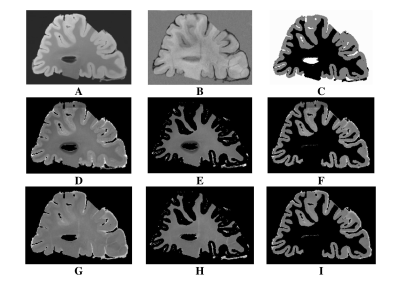
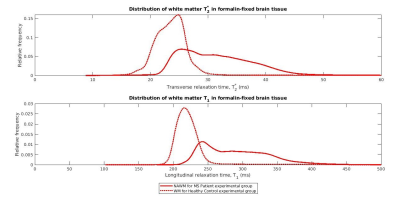
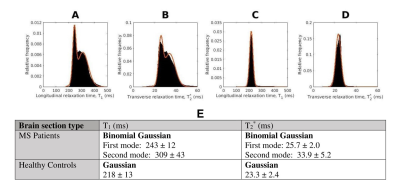
Data Processing: For each 3D-MP2RAGE measurement, a T1 map was computed using Siemens’ MP2RAGE-based T1 fitting algorithm. Individual T1 maps and ME-GRE magnitude images were averaged to yield high SNR T1 maps and ME-GRE images for each brain section. The averaged T1 data was upsampled and registered to the ME-GRE data using FLIRT (FSL, v7.2). Based on averaged ME-GRE magnitude data, T2* maps were computed using a voxel-wise non-linear, least-squares fitting approach in MATLAB (R2020b, Mathworks). To extract tissue-specific T1 and T2* maps, the first echo image of the averaged ME-GRE was used to segment GM and WM, via the ilastik Pixel Classification workflow5 (Figure 1). Next, normalized, smoothed T1 and T2* distributions in WM were generated from the MS patient and healthy control experimental groups (Figure 2). The distributions were fitted with bimodal and unimodal Gaussian functions to extract unique peak positions (Figure 3). Given the bimodal T2* and T1 distributions for the MS patient group, the first (lower) and second (higher) modes were assumed to arise from the presence of NAWM and DAWM respectively. This assumption was made since DAWM is expected to entail a greater increase in T2* and T1 compared to NAWM. To that end, “NAWM” here refers to all non-plaque WM outside the region classified as DAWM.
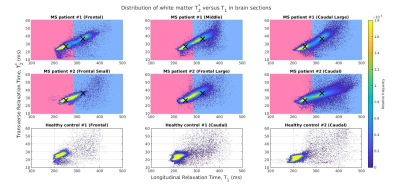
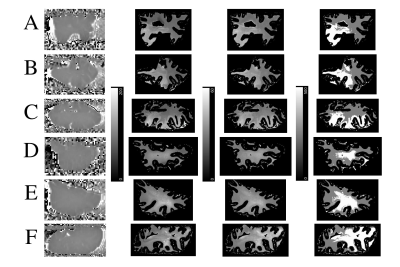
Classification: K-means clustering (k = 2) was applied to the MS tissue T1 and T2* 2D data to create a classifier capable of discriminating between apparent NAWM and DAWM. For each brain section, a bivariate histogram of T2* versus T1 was plotted (Figure 4) and clusters of T2*-T1 values representative of DAWM (blue) and NAWM (pink) were indicated. A mask showing the spatial distribution of DAWM was also generated (Figure 5).
Results and Discussion
T2* and T1 distributions in the MS patient group were lower in peak height, broader and shifted to longer relaxation time values compared to those of the healthy control group (Figure 3E). The broader distributions suggest heterogeneity of non-plaque abnormal WM6. The difference in NAWM and DAWM T1 and T2* peak positions reflects the impact of myelin phospholipid damage3 on DAWM.Bivariate T2*-T1 relative frequency histograms (Figure 4) revealed two distinct hyperintensities in MS tissue which were not present in control tissue. The hyperintensities were demarcated by a linear decision boundary (approximately T1 = 289 ms) calculated with K-means clustering. Mean (T1, T2*) positions for the k-means clusters representative of NAWM and DAWM were (250, 26.7)ms and (325, 36.1)ms respectively.
The DAWM regions detected by the K-means classifier were located in periventricular WM (Figure 5), consistent with results of MS histopathological studies3. In most MS sections (A, B, F), DAWM did not have ill-defined borders but, rather, was well-delineated, comprising nearly half the tissue slab area. These findings support the joint use of 7 T T2* and T1-based relaxometry approaches for identifying NAWM and DAWM pathology in MS. More definitive support based on ex-vivo histological myelin staining is underway, as a topic of our future work.
Acknowledgements
We gratefully acknowledge the Douglas-Bell Canada Brain Bank as the source of formalin fixed cerebral human brain tissue used in our work.References
1. Lassmann H, van Horssen J Fau - Mahad D, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. (1759-4766 (Electronic)).
2. Stüber C, Morawski M, Schäfer A, et al. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage. 2014;93 Pt 1:95-106.
3. Moore GR, Laule C, Mackay A, et al. Dirty-appearing white matter in multiple sclerosis: preliminary observations of myelin phospholipid and axonal loss. J Neurol. 2008;255(11):1802-1811.
4. Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134(12):3602-3615.
5. Berg S, Kutra D, Kroeger T, et al. ilastik: interactive machine learning for (bio)image analysis. Nature Methods. 2019;16(12):1226-1232.
6. Vrenken H, Geurts JJ, Knol DL, et al. Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology. 2006;240(3):811-820.
Figures