3957
Initial in vivo evidence of fibrin deposition in multiple sclerosis patients using fibrin-targeted 64Cu-FBP8 positron emission tomography1Radiology, Massachusetts General Hospital; A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Neurology, Massachusetts General Hospital, Boston, MA, United States, 4Neurology, Beth Israel Deaconess Medical Center, Boston, MA, United States
Synopsis
In multiple sclerosis, experimental and histopathological studies point to fibrinogen as a link between a damaged brain blood barrier and the initiation of the inflammatory demyelination and neurodegeneration in both cortex and white matter. Nevertheless, although the widespread accumulation of fibrinogen as fibrin deposits within multiple sclerosis lesions is well documented on postmortem brain tissue, in vivo evidence is still lacking. Using a novel fibrin-specific positron emission tomography probe, 64Cu-FBP8, we were able to demonstrate in vivo fibrin deposition not only in active white matter multiple sclerosis lesions but also in the cortex of a few multiple sclerosis patients.
Purpose
In multiple sclerosis (MS) increasing evidence highlights a central role for fibrinogen, a protein of the coagulation cascade, whose leakage across a disrupted blood brain barrier and subsequent accumulation within CNS as fibrin promotes inflammatory demyelination, neuronal damage and inhibits tissue repair processes1. Moreover, fibrin deposition is not only an early feature of acute MS pathology, since it persists throughout disease stages and can be found even in some chronic white matter (WM) lesions and in cortex despite the lack of contrast enhancement on magnetic resonance imaging (MRI)2-5. Nevertheless, the evidence of fibrin deposition in MS beyond postmortem immunohistochemical analyses has not been achieved yet. This study aims to translate a new fibrin-targeted 64Cu-FBP8 positron emission tomography probe developed by Massachusetts General Hospital investigators for in vivo imaging of fibrin deposition in the brain of MS patients.Methods
Image acquisition and analysis: Preliminary data were obtained in 4 MS patients (3 SPMS and 1 RRMS, aged between 46-54 years) and 2 age-matched controls (patients with cardiac disease but without pathological brain changes or history of brain disease) that underwent 140 minutes of 64Cu-FBP8 simultaneous MR-PET. During this session were also acquired: 3T multiple gradient echoes 3D-magnetization prepared rapid acquisition scans (MEMPRAGE, 1 mm isotropic) for cortical surface reconstruction (using Freesurfer) and PET image registration; 3T fluid-attenuated inversion recovery scans (1mm isotropic) for WM lesion segmentation (using Slicer 4.4). Standardized uptake value (SUV) maps were created for the 20 minutes PET frame, starting 120 minutes post-injection and then, they were normalized (SUVR) to take into account global differences across subjects, by a pseudo-reference region with SUV levels similar in MS and controls. At the end of the PET session post gadolinium MEMPRAGE scans were obtained for identification of possible areas of lesion enhancement in the WM and cortex. Statistical analysis: Differences between groups were assessed by linear regression models including age as a covariate of no interest.Results
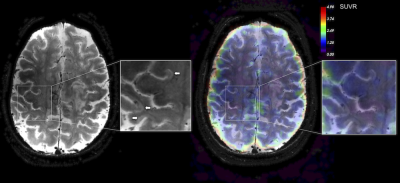
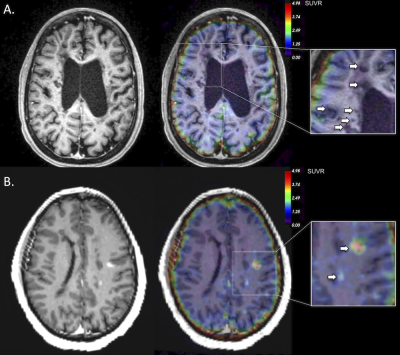
Illustrative 64Cu-FBP8 SUVR map in a SPMS patient is shown in Figure 1. Overall, our results demonstrate an increased (by 46.5%) 64Cu-FBP8 SUVR uptake in the MS cortex relative to controls (mean±SD=2.7±0.7 versus 1.8±0.5, p=0.04). Other gray matter structures of abnormal increased radiotracer uptake in MS patients as compared to controls included: basal ganglia (1.2±0.4 versus 0.8±0.1, p<0.001); hippocampus (2.2±0.7 versus 1.5±0.4, p=0.02) and thalamus (1.3±0.3 versus 1±0.2, p=0.04). In MS normal appearing WM SUVR tended to be higher (by 51.9%) than in controls (mean±SD=1.5±0.4 versus 1±0.2, p=0.06). In the 422 chronic WM lesions we were not able to detect any significant increase in the SUVR values (Figure 2A). In contrast, in the 4 active lesions identified in the RRMS patient, 64Cu-FBP8 SUVR were significantly increased (by 109%, mean±SD=2.1±1.6, Figure 2B).Discussion and Conclusion
Using 64Cu-FBP8 MR-PET our data demonstrate in vivo fibrin deposition not only in the active WM lesions, but also in the MS cortex. If the presence of fibrin in enhancing lesions could be related to an open brain blood barrier, abnormal endothelial tight junctions, that allow a very subtle, but possibly permanent fibrinogen extravasation could explain cortical fibrin deposition. Fibrin deposition in the MS cortex was confirmed by previous postmortem examinations that also suggested a role for fibrin deposition in cortical neurodegenerative events in MS6. Further studies are needed to see if the in vivo quantification of fibrin deposition by 64Cu-FBP8 could be used to track the evolution of parenchymal damage in MS lesions and to which extent, the amount of fibrin deposition relates to tissue demyelination and neurodegeneration in the cortex and WM.Acknowledgements
NMSS PP-1812-32987References
1. Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nature reviews Neuroscience 2018;19:283-301.
2. Vos CM, Geurts JJ, Montagne L, et al. Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiology of disease 2005;20:953-960.
3. Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. The Journal of pathology 2003;201:319-327.
4. Kwon EE, Prineas JW. Blood-brain barrier abnormalities in longstanding multiple sclerosis lesions. An immunohistochemical study. Journal of neuropathology and experimental neurology 1994;53:625-636.
5. Davalos D, Ryu JK, Merlini M, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nature communications 2012;3:1227.
6. Yates RL, Esiri MM, Palace J, Jacobs B, Perera R, DeLuca GC. Fibrin(ogen) and neurodegeneration in the progressive multiple sclerosis cortex. Ann Neurol 2017;82:259-270.
Figures