3953
Wireless Physiological Motion Monitoring with an Integrated RF/Wireless Coil Array and MR-Compatible Ultrasound-Based System1Brain Imaging And Analysis Center, Duke University, Durham, NC, United States, 2Medical Physics Graduate Program, Duke University, Durham, NC, United States, 3Brain Imaging And Analysis Center, GE Healthcare, Aurora, OH, United States, 4Radiology, Brigham and Women's, Harvard Medical School, Boston, MA, United States
Synopsis
A wireless MR-compatible ultrasound-based device consisting of an integrated RF/wireless coil, organ-configuration motion sensor, Raspberry Pi, pulser/receiver, and analog-to-digital converters was developed to acquire, digitize, and wirelessly transmit ultrasound data while simultaneously acquiring MR-images. Experiments were performed to demonstrate the system's 1) mobility through continuously acquiring, digitizing, and wirelessly transmitting ultrasound data at multiple locations, and 2) ability to wirelessly monitor physiological motion during coughing/gasping. This integrated system enables the OCM sensor to travel with patients throughout the hospital while also demonstrating the ability of the iRFW coil and backend electronics to process and wirelessly transmit a high-fidelity signal.
Introduction
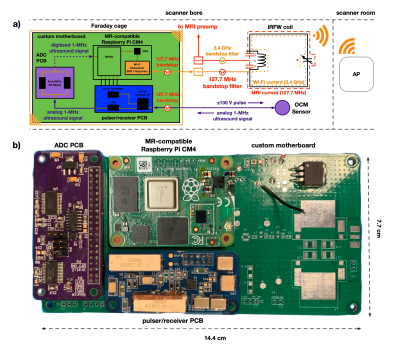
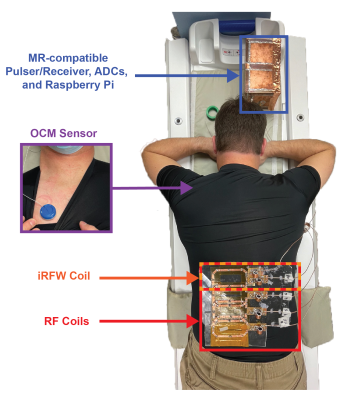
Physiological motion can significantly degrade MR image quality and/or therapeutic treatment accuracy. Most MR-compatible physiological motion monitoring systems (e.g., navigator echoes and optical tracking) are confined to a given scanner/treatment room, which limits their ability to provide uninterrupted motion tracking across multiple imaging/treatment procedures in the patient workflow. In contrast, small (~3x3x1 cm) ultrasound-based devices called organ-configuration motion (OCM) sensors, which attach to the skin and can move with the patient, allow continuously acquired ultrasound data to be used to monitor and/or correct for physiological motion across multiple procedures. This information can be used in combination with machine learning algorithms to correct motion artifacts, aid in image registration, and/or detect irregular physiological motion, such as coughing or gasping. However, OCM sensors require many wired electronics (e.g., pulser/receiver, analog-to-digital converters (ADC), CPU) that need to be moved with the patient, which limit their mobility1,2.The recently proposed integrated RF/wireless (iRFW) coil design3-5 can simultaneously perform MR imaging and wireless data transfer by allowing RF currents at the Larmor frequency and in a wireless communication band to flow on the same coil element. This coil design was integrated with an OCM sensor to enable wireless ultrasound data transmission in the scanner bore6. However, this system did not provide full OCM mobility because the MR-incompatible pulser/receiver had to remain in the machine room, connected with cables. To address this limitation, we designed and integrated a 4-channel iRFW coil array with a new completely wireless MR-compatible ultrasound device (12.8 x 12.8 x 20.4 cm), which can acquire, digitize, and wirelessly transmit ultrasound data throughout different stages of patient care. In vivo experiments are performed to demonstrate the system’s ability to perform MR imaging and wireless ultrasound data transmission at multiple locations in the hospital.
The present work fits in a tradition of hybrid MRI/ultrasound imaging methods7-11, and compared to prior approaches it emphasizes ease-of-use. OCM sensors are small and simply attach to the skin, without the need for any extra hardware to hold/orient the ultrasound transducer. By making OCM hardware wireless and contained in a small MR-compatible box, a further step is taken here toward making hybrid MRI/ultrasound imaging practical and convenient.
Methods
First, a 5x7-cm iRFW coil element (Fig. 1, Fig 2) was constructed and tuned on a vector network analyzer to be resonant at the Larmor frequency (127.7 MHz for GE 3T scanners) and in a Wi-Fi frequency band (2.412-2.472 GHz) for simultaneous MR signal reception and wireless ultrasound data transmission. Three conventional RF coil elements of similar size were constructed to make a 4-channel coil array (Fig. 2), with the proper overlap to decouple adjacent coil elements and maintain SNR.A wireless MR-compatible ultrasound device (Fig. 1, Fig. 2) was created by designing a custom pulser/receiver printed circuit board (PCB), which could provide a 105-V excitation pulse to the OCM sensor (Fig. 1, Fig. 2) and receive the returned 1-MHz ultrasound signal. Next, a custom ADC PCB was created4 to digitize the ultrasound signal in preparation for wireless data transmission. Finally, these two PCBs were connected via a custom motherboard to a modified Raspberry Pi Compute Module, which was used to control the device and wirelessly transfer the ultrasound data from an on-board Wi-Fi module via the iRFW coil element to access points (APs) on a private Wi-Fi network.
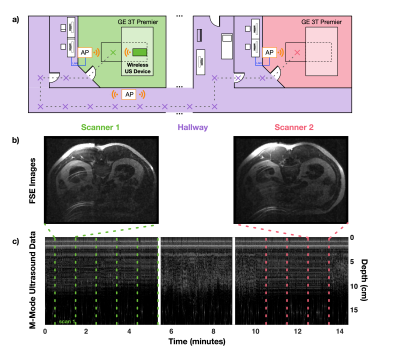
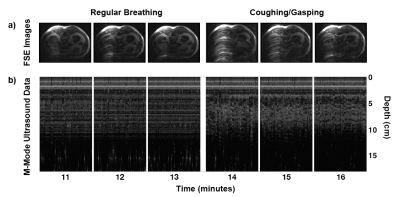
Two in vivo experiments were performed to demonstrate the system's mobility and ability to monitor physiological motion. Specifically, T1-weighted fast spin-echo (FSE) abdominal images were acquired on a healthy volunteer while simultaneously acquiring, digitizing, and wirelessly transmitting ultrasound data from an OCM sensor placed on the subject’s chest (Fig. 2). Next, the coil array was disconnected from the scanner and both the iRFW-OCM system and subject were moved on the patient table through a hallway to a second MRI scanner where the same scans were repeated, all while acquiring, digitizing, and wirelessly transmitting ultrasound data (Fig. 3a). In the second experiment, the subject was asked to breathe regularly in the first three scans and to cough in the final three scans to demonstrate the system’s ability to detect different breathing motions.
Results and Discussion
The M-Mode ultrasound data acquired continuously throughout the first experiment (Fig. 3c) with the corresponding FSE images acquired at both scanners (Fig. 3b) demonstrate the system’s mobility and ability to perform simultaneous wireless ultrasound data transmission and MR image acquisition. Furthermore, the M-Mode ultrasound data acquired continuously throughout the second experiment (Fig. 4b) with the corresponding time series of simultaneously-acquired FSE images (Fig. 4a) demonstrate the system’s ability to monitor respiratory motion wirelessly, as shown by the irregular patterns in the M-Mode ultrasound data and the more severe breathing artifacts in the FSE images.Conclusion
These in vivo experiments demonstrate the mobility of the wireless ultrasound device and its ability to continuously monitor physiological motion during various stages of patient care. More specifically, the ultimate goal of this project involves using these sensors to help bridge, fuse or otherwise link procedures that occur at different times/places in the course of a patient’s treatment, for example MRI, PET and/or tumor ablation procedures.Acknowledgements
This work was in part supported by NIH grants P41EB015898 and R01EB030470.References
1. Madore B, Preiswerk F, Bredfeldt JS, Zong S, Cheng CC. Ultrasound-based sensors to monitor physiological motion. Med Phys 2021;48(7):3614-3622.
2. Preiswerk F, Toews M, Cheng CC, Chiou JG, Mei CS, Schaefer LF, Hoge WS, Schwartz BM, Panych LP, Madore B. Hybrid MRI-Ultrasound acquisitions, and scannerless real-time imaging. Magn Reson Med 2017;78(3):897-908.
3. Darnell D, Cuthbertson J, Robb F, Song AW, Truong TK. Integrated radio-frequency/wireless coil design for simultaneous MR image acquisition and wireless communication. Magn Reson Med 2019;81(3):2176-2183.
4. Cuthbertson JD, Darnell D, Stormont R, Robb F, Song AW, Truong TK, 2019. A 4-channel iPRES-W AIR coil array for simultaneous MR image acquisition and wirelessly-controlled localized B0 shimming of the spinal cord. Proc ISMRM 27, 1489.
5. Cuthbertson JD, Truong TK, Yadav, V, Robb F, Song AW, Darnell D, 2020. Dual-Stream iPRES-W Head Coil Array for MR Imaging, Wireless Respiratory Tracking, and Wireless Localized B0 Shimmed. Proc ISMRM 28, 1262.
6. Willey D, Bresticker J, Truong T-K, Song A, Madore B, Darnell D. Integrated RF/Wireless Coil and Ultrasound-Based Sensors to Enable Wireless Physiological Motion Monitoring in MRI. 2020; Virtual Meeting, p. 1282.
7. Günther M, Feinberg DA. Ultrasound-guided MRI: preliminary results using a motion phantom. Magn Reson Med 2004;52(1):27-32.
8. Arvanitis CD, Livingstone MS, McDannold N. Combined ultrasound and MR imaging to guide focused ultrasound therapies in the brain. Phys Med Biol 2013;58(14):4749-4761.
9. Bednarz BP, Jupitz S, Lee W, Mills D, Chan H, Fiorillo T, Sabitini J, Shoudy D, Patel A, Mitra J, Sarcar S, Wang B, Shepard A, Matrosic C, Holmes J, Culberson W, Bassetti M, Hill P, McMillan A, Zagzebski J, Smith LS, Foo TK. First-in-human imaging using a MR-compatible e4D ultrasound probe for motion management of radiotherapy. Phys Med 2021;88:104-110.
10. Kording F, Yamamura J, de Sousa MT, Ruprecht C, Hedstrom E, Aletras AH, Ellen Grant P, Powell AJ, Fehrs K, Adam G, Kooijman H, Schoennagel BP. Dynamic fetal cardiovascular magnetic resonance imaging using Doppler ultrasound gating. J Cardiovasc Magn Reson 2018;20(1):17.
11. Petrusca L, Cattin P, De Luca V, Preiswerk F, Celicanin Z, Auboiroux V, Viallon M, Arnold P, Santini F, Terraz S, Scheffler K, Becker CD, Salomir R. Hybrid ultrasound/magnetic resonance simultaneous acquisition and image fusion for motion monitoring in the upper abdomen. Investigative radiology 2013;48(5):333-340.
Figures