3949
iMPI – first real-time imaging with a human-sized interventional Magnetic Particle Imaging scanner1Experimental Physics 5 (Biophysics), University of Würzburg, Würzburg, Germany, 2Diagnostic and Interventional Neuroradiology, University Hospital Würzburg, Würzburg, Germany, 3Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany
Synopsis
In this work a first human-sized Magnetic Particle Imaging (MPI) scanner designed and specifically engineered for experimental (cardio)vascular interventions is presented.
Based on a novel open design implementing the so called traveling wave MPI approach, the open iMPI system provides imaging of Tracers based on superparamagnetic iron oxide nanoparticles (SPIONs) with high sensitivity, optimal patient handling and would even allow hybrid imaging of magnetic tracers within gold standard x-ray based interventional angiography systems.
In initial experiments, the feasibility of a human-sized interventional MPI scanner with real-time data reconstruction and image visualization is demonstrated.
Introduction
Over the past decade, Magnetic Particle Imaging (MPI) has become a promising tomographic method for numerous experimental applications in biology, chemistry, physics and medicine [1]. For cardiovascular medicine in particular, MPI could become an applicable radiation-free technique for image-guided endovascular interventions supporting the common x-ray gold-standard (digital subtraction angiography, DSA) in the future [2, 3].On the way to potentially clinical applications, several studies have demonstrated the feasibility of using MPI scanners in preclinical scenarios [4]. In-vivo experiments in rodents [5] demonstrated the potential of MPI with all its features, such as radiation-free imaging with high spatial [6] and high temporal resolution [7], as well as very high tracer sensitivity [8] and specificity [9].
As a direct tomographic method, MPI relies on the non-linear magnetization response of superparamagnetic iron-oxide nanoparticles (SPIONs) on time-varying magnetic fields. Novel ways to synthetize MPI-tracers improve image quality and acquisition time and/or reduce hardware requirements [10, 11].
The next step is to upgrade MPI hardware for human-scale applications [12, 13]. This presents some challenges when trying to maintain the flexibility and speed of MPI. In addition, issues related to specific absorption rate (SAR) and peripheral nerve stimulation (PNS) limitations [14] due to strong magnetic field gradients required for high spatial resolution have to be considered.
In this abstract, the first real-time imaging results using a human-sized MPI scanner dedicated for vascular interventions based on the Traveling Wave approach are presented [15]. The iMPI scanner was specifically designed to meet the requirements for real-time vascular interventions such as percutaneous transluminal angioplasty (PTA) and stenting, particularly for human extremities.
Methods
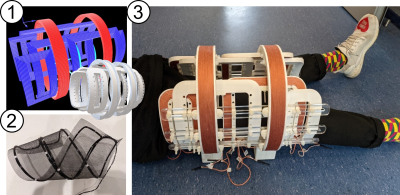
The aim of the interventional MPI scanner (iMPI) project is to provide a radiation-free system that can meet similar requirements as clinical angiography units. This requires submillimeter spatial resolution, high temporal resolution of at least 5 images per second, near real-time visualization with latency below 100 ms [16, 17], and an open design that provides a comfortable and flexible environment for patients and medical staff, as well as sufficient space for interventional instrumentation and its operation (see Fig. 1). Additionally, the open design should allow simultaneous performance of conventional DSA, which is especially important during trial and testing phase [18].To generate a sufficient magnetic field gradient required for a high spatial resolution in MPI, a novel hardware approach is used to generate a field free line (FFL) dynamically within a specific region of interest (ROI). By rapidly moving the FFL along specific trajectories through the ROI using additional magnetic fields, the tracer signal is inductively measured and used to determine the spatial SPION distribution. To ensure high signal-to-noise-ratio (SNR) even with a single shot-sequence, the receive coil has been designed as a flexible mesh-like gradiometric coil system (fig. 1 (2)), which can be positioned as close as possible to the ROI. The imaging result of e.g. vascular structures and interventional instruments is a projection display comparable to conventional DSA.
Results
In initial experiments, a magnetic field gradient of about 0.5 T/m could be achieved, which is sufficient for a spatial resolution of about 10 mm when the system is operated at the maximum imaging frequency of at least 10 frames per second and currents of about 150 Ampere. This results in a power dissipation of about 50 Kilowatts in continuous mode, requiring a sophisticated cooling management.An alternative approach is a pulsed measurement mode, where a short sequence (<1 ms) is generated to sequentially scan the ROI and avoid overheating the system.
Fig. 2 shows a time series of a small sample moving and rotating in the iMPI. The duty cycle of the sequence was 5% (20 ms signal acquisition for one full projection) and the data has been reconstructed and visualized in real-time (<100 ms latency).
Discussion
Since the main idea of the iMPI device is to support clinical angiography systems, the scanner should have an open design to ensure a clear view through the system as well as good patient handling. This means that the iMPI scanner does not have complete shielding, which reduces SNR drastically. To overcome this issue, a novel transmit-receive combination had to be built [8] and more sophisticated data processing had to be implemented to subsequently reduce noise in the data [19].Conclusion
A first projection MPI scanner fitting a human leg has been designed and built, providing promising results that may open up new possibilities on the way to clinical applications.Acknowledgements
Grant support by the Deutsche Forschungsgemeinschaft (DFG) under grant number VO 2288/1-1 is greatly acknowledged.References
[1] Gleich B & Weizenecker J. Tomographic Imaging using the nonlinear response of magnetic particles. Nature. 2005; 435:1214-7.
[2] Herz S et al. Magnetic Particle Imaging Guided Real-Time Percutaneous Transluminal Angioplasty in a Phantom Model. Cardiovasc Intervent Radiol. 2018; 41(7):1100-5.
[3] Herz S et al. Magnetic Particle Imaging-Guided Stenting. J Endovasc Ther. 2019; 26(4):512-9.
[4] Chandrasekharan P et al. A perspective on a rapid and radiation-free traver imaging modality, magnetic particle imaging, with promise for clinical translation. Br J Radiol. 2018; 91:20180326.
[5] Vogel P et al. First in-vivo Traveling Wave Magnetic Particle Imaging of a beating mouse heart. Phys Med Biol. 2016; 61(18):6620-34.
[6] Vogel P et al. Micro-Traveling Wave Magnetic Particle Imaging – sub-millimeter resolution with optimized traver LS-008. IEEE TMAG. 2019: 55(10):5300207.
[7] Vogel P et al. Superspeed Bolus Visualization for Vascular Magnetic Particle Imaging. IEEE TMI. 2020; 39(6):2133-9.
[8] Graeser M et al. Towards Picogram Detection of Superparamagnetic Iron-Oxide Particles Using a Gradiometric Receive Coil. Sci-Rep. 2017; 7:6872.
[9] Wu K et al. Magnetic Particle Spectroscopy for Detection of Influenza A Virus Subtype H1N1. ACS Appl Mater Interfaces. 2020; 12:13686-97.
[10] Tay Z W et al. Superferromagnetic Nanoparticles Enable Order-of-Magnitude Resolution & Sensitivity in Magnetic Particle Imaging. Small Methods. 2021; 2100796.
[11] Vogel P et al. SynoMag®: The New High-Performance Tracer for Magnetic Particle Imaging. Int J on Magn Part Imaging. 2021; 81:210-4.
[12] Graeser M et al. Human-sized magnetic particle imaging for brain applications. Nature Comm. 2019; 10:1936.
[13] Mason E E et al. Design analysis of an MPI human functional brain scanner. Int J Magn Part Imaging. 2017; 3(1):1703008.
[14] Saritas E U. Magnetostimulation Limits in Magnetic Particle Imaging. IEEE TMI. 2013; 32(9):1600-10.
[15] Vogel P et al. Traveling Wave Magnetic Particle Imaging. IEEE TMI. 2014; 33(2):400-7.
[16] Vogel P et al. Flexible and Dynamic Patch Reconstruction for Traveling Wave Magnetic Particle Imaging. Int J Magn Part Imaging. 2016; 2(2):1611001.
[17] Vogel P et al. Low Latency Real-time Reconstruction for MPI Systems. Int J Magn Part Imaging. 2017; 3(2):1707002.
[18] Vogel P et al. Magnetic Particle Imaging meets Computed Tomography: first simultaneous imaging. Nature Sci Rep. 2019; 9:12627.
[19] Srinivas S A et al. External Dynamic InTerference Estimation and Removal (EDITER) for low field MRI. MRM. 2021; early view.
Figures