3945
Combining gradient and spin echo calibrated fMRI for mapping brain oxygen extraction fraction1School of Psychology, Cardiff University, Cardiff, United Kingdom, 2School of Physics and Astronomy, Cardiff University, Cardiff, United Kingdom, 3Department of Neuroscience, University “G. d'Annunzio” of Chieti-Pescara, Chieti, Italy, 4Institute for Advanced Biomedical Technologies, University “G. d'Annunzio” of Chieti-Pescara, Chieti, Italy
Synopsis
We propose a new calibrated fMRI approach for non-invasive measurement of the cerebral metabolic rate of oxygen of oxygen consumption and the deoxyhaemoglobin weighted blood volume. The method exploits the differential dependence of the gradient and spin echo BOLD signals on OEF. Unlike conventional calibrated approaches, we show that gradient-spin echo calibrated fMRI requires only a single respiratory modulation to determine OEF. The method is applied in-vivo using a repeated breath-holding protocol, and initial results show agreement with global measurements of oxygen saturation.
Introduction
Magnetic resonance imaging (MRI) offers the possibility to non-invasively map the rate of cerebral metabolic oxygen consumption (CMRO2) and oxygen extraction fraction (OEF). Existing methods, based on respiratory modulation of arterial spin labelling (ASL) and blood oxygen level dependent (BOLD) signals, require lengthy acquisitions and independent modulation of both arterial oxygen and carbon dioxide levels. We present a new method that combines gradient and spin echo readouts with ASL data acquisition and only a single respiratory manipulation (GSE-calibrated fMRI). The proposed method has the potential to significantly simplify data acquisition compared to existing dual-calibrated fMRI methods.Monte-Carlo simulations were used to create multi-compartment signal models of gradient and spin echo BOLD responses to changes in blood susceptibility. The model predicts an offset in the intercept of the BOLD signal change vs OEF (oxygen extraction fraction) in the gradient echo response compared to the spin echo response. Thus, the ratio between the two BOLD responses is dependent on OEF. We propose to measure OEF and deoxyhaemoglobin weighted blood volume (CBVdHb) by combining a measure of flow change with the gradient and spin echo responses to hypercapnia
Methods
The ODIN framework1 was used to simulate extravascular R2 and R2* surrounding a cylinder in a 2D plane with a defined radius and susceptibility. Each cylinder (vessel) was modelled independently, while random vessel orientations were accounted for by varying the angle between the main field and the cylinder in the range from 0 to π/2. Simulated vessel radii (4μm and 20μm) were chosen to reflect in-vivo reports of capillary (4μm), mean arteriolar (17.8μm) and mean venular (23.3μm) radii 2,3. The vessel simulations were incorporated into multi-compartment vascular models following the method of Uludag et al 2009 4, which incorporates empirical observations of intravascular R2 and R2* determined at 3 Tesla. Cerebral vascular blood compartments are thought to compose of approximately 20-30% arterial, 30-45% capillary, and 40-50% venous fractions 5,6. In our model we define a parameter ρ, which represents the ratio of CBVdHb / capillary blood volume (where CBVdHb includes both the capillary and venous fractions).Pilot data was collected in six healthy volunteers (mean age 27.8 ± 12 years, 2 female). Data were acquired using a 3T Siemens MAGNETOM Prisma. An 8 minute 45 second dual-excitation pseudo-continuous arterial spin labelling and BOLD-weighted acquisition was acquired during repeated breath-holding (20 second breath-hold and 30 second recovery with paced breathing). A separate spin echo acquisition was acquired with the same respiratory protocol immediately following the BOLD/ASL acquisition. TRUST 7 data was acquired for global estimation of OEF. MRI data fitting was performed with a constrained minimisation routine. It was assumed that similar flow changes occurred in the gradient and spin echo breath-holding acquisitions.
Results
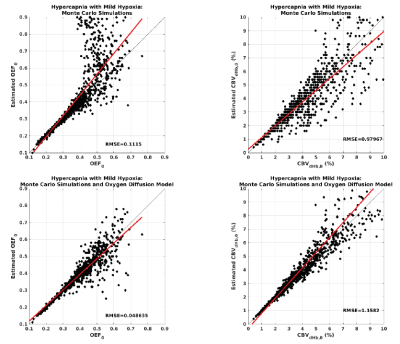
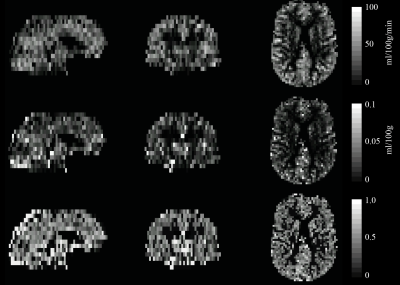
Simulations performed with the vascular model show that when ρ is within reported physiological bounds, OEF and CBVdHb can be determined using an iso-metabolic flow stimulus e.g., hypercapnia. Figure 1 shows the simulated spin echo and gradient echo BOLD signal responses for typical hypercapnia and breath-hold (hypercapnia with mild hypoxia) experiments. The simulations include a coefficient of variation of ρ of 0.2, with all other simulation parameters fixed and assumed to be measured. In this example, the ratio of the spin echo to gradient echo signal uniquely determines OEF up until an OEF of approximately 0.5 to 0.6 where the plots plateau out. Simulating over the complete parameter space of expected physiology for a breath-holding experiment, figure 2, confirms this observation with a good correlation between the known and estimated (from model inversion) OEF up to the observed plateau region. However, by restricting parameter estimates with a simple model of oxygen diffusion 8, the accuracy increases (RMSE reduces from 0.1 to 0.05) and range of viable estimation is also increased (figure 2 bottom row). The pilot dataset demonstrates the promise of the method. Figure 3 shows example OEF and CBVdHb maps collected in one subject. The mean grey matter OEF across the 6 pilot datasets was 0.49 ± 0.07, compared to 0.35 ± 0.06 in the global TRUST measurement. The mean CBVdHb estimate was 3.1 ± 0.2 ml/100g in grey matter. The GSE-calibrated measurements correlate with the global OEF measurements (R2 = 0.52) although not significantly in this small pilot cohort. The higher OEF estimated with the calibrated approach could be physiological, as the baseline perfusion value calculated during the repeated breath holds appears to be depressed (mean grey matter perfusion = 38 ± 5.9 ml/100g/min).Discussion/Conclusion
We have proposed a new method for calibrated measurement of absolute CMRO2, that requires only one respiratory challenge. The method exploits the differential OEF dependence of the gradient echo and spin echo response to an isometabolic flow increase. Simulations predict low uncertainty when OEF is below approximately 0.4. Thus, the method should be well suited to detecting regional reductions in OEF. The uncertainty for higher OEF values can be reduced by including constraints based on a simple model of oxygen diffusion in the capillary bed. Initial results using a repeated breath holding challenge suggest good agreement with global measures of OEF. Future work will apply concurrent GE/SE readouts to increase the robustness and sensitivity of the method.Acknowledgements
The study was funded by the Wellcome Trust UK and the Engineering and Physical Sciences Research CouncilReferences
1. T.H. Jochimsen and M. von Mengershausen. ODIN-object-oriented development interface for NMRJ. Magn. Reson., 2004; 170: 67-78
2. Saiga R., et al. Brain capillary structure of schizophrenia cases and controls show a correlation with their neuron structures. Scientific Reports. 2021: 11, Article number: 11768
3. Adams DL., et al. Vascular Supply of the Cerebral Cortex is Specialized for Cell Layers but Not Columns. Cereb Cortex. 2015; 25(10):3673-81
4. Uludag K, Muller-Bierl B, Ugurnil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage. 2009: 5;48(1):150-65
5. Van Zijl et al. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nature Medicine. 1998; 4: 159–167
6. Weber B et al. The Microvascular System of the Striate and Extrastriate Visual Cortex of the Macaque. Cereb Cortex. 2008; 18(10):2318-3
7. Lu H and Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008; 60(2):357-63
8. Germuska et al. A Frequency-Domain Machine Learning Method for Dual-Calibrated fMRI Mapping of Oxygen Extraction Fraction (OEF) and Cerebral Metabolic Rate of Oxygen Consumption (CMRO2). Front Artif Intell. 2020; 3: 12.
Figures