3934
Rapid Low SAR T2 Relaxometry near Metallic Implants1Siemens Medical Solutions USA, New York, NY, United States, 2Department of Radiology, New York University School of Medicine, New York, NY, United States, 3Department of Orthopedic Surgery, New York University School of Medicine, New York, NY, United States
Synopsis
Quantitative T2 mapping in the presence of metal implants is affected by susceptibility-related artifacts. We propose a low flip angle view angle tilting-enabled turbo-spin echo sequence along with spatiotemporal undersampling for low SAR scan efficient T2 mapping. The proposed technique would allow 2.5min T2 mapping with high temporal sampling of the T2 relaxation curve. Performance of the proposed technique is evaluated using retrospective under-sampling simulations and phantom experiments using a cobalt-chromium hip arthroplasty implant.
Background and Motivation
Quantitative T2 characterization of musculoskeletal tissues permits the detection of cartilage and tendon degeneration before morphologically visible substance defects occur[1-3]. In clinical practice, T2 relaxation values are frequently obtained by acquiring T2-weighted images at multiple echo times (TE). While MRI techniques for T2 quantification are sufficiently accurate in the native human body[4], quantitative MRI in the presence of metal implants is affected by susceptibility-related artifacts.T2 mapping near metal implants has been the focus of a few recent studies[5-7]. These studies make use of metal artifact reduction sequences (MARS) to sample the T2 relaxation curve. However, due to scan time considerations, these approaches typically acquire data at only two echo times. In addition, the acquired data is fit to a mono-exponential model that does not account for the effect of stimulated echoes from slice profile imperfections.
In this work, we propose a low flip angle view angle tilting (VAT)-enabled turbo-spin echo (TSE) sequence along with spatiotemporal undersampling for low specific absorption rate (SAR) scan efficient T2 mapping. The proposed technique is evaluated using retrospective under-sampling simulations and phantom experiments using a cobalt-chromium hip arthroplasty implant.
Theory
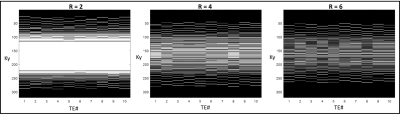
A VAT-enabled TSE sequence[8] was used to acquire data at multiple TEs to sample the T2 decay curve for accurate T2 mapping. To accelerate the acquisition, we propose using spatiotemporal undersampling with a variant of the complementary Poisson disc scheme [9]. Figure 1 shows the k-t sampling pattern for 2D Cartesian imaging at three different acceleration rates. Note that this scheme samples the center of k-space at each TE, thereby reliably encoding the T2-weighted contrast.The undersampled data is reconstructed using a subspace constrained algorithm[10]: $$\hat{M} = arg min_M ||\sum_i^NE_i \Psi M -K_i||_2^2+\lambda R(M)$$
where, $$$E$$$ is the Fourier encoding operator, $$$K$$$ is the acquired k-space data, $$$\Psi$$$ is a $$$L$$$-dimensional principal component subspace basis such that $$$L <<< ETL$$$ and $$$M$$$ are the principal component coefficients. $$$R$$$ is a locally low-rank regularization term with scaling factor $$$\lambda$$$. The subspace basis $$$\Psi$$$ is generated using a Bloch equation-based model of the TSE sequence[11]. The reconstruction was implemented using an alternating direction method of multipliers algorithm.
We also propose the use of low flip angles for the excitation and refocusing RF pulses in the TSE sequence to minimize SAR. However, this requires the use of a Bloch equation-based instead of monoexponential-fitting for accurate T2 estimation.
Phantom Imaging
All experiments were performed at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). A metal-on-metal cobalt-chromium hip arthroplasty implant was embedded in a background of doped water solution. A set of T2 phantoms with T2 values ranging from 30ms–70ms were taped to the femoral component of the implant.All data were acquired using a high transmit-receive bandwidth TSE sequence equipped with 100% VAT with the following parameters: resolution=0.93mmx0.93mmx3mm, ETL=9, echo-spacing=6ms, and the following excitation-refocusing flip angle combinations: [ex:30o, ref:60o], [ex:30o, ref:90o], [ex:45o, ref:105o]. The following TEs were acquired for T2 mapping: [5.7, 11, 17, 23, 34, 40, 51, 63, 74, 80] ms. The acquisition was repeated using the same set of T2 phantoms without the metal implant.
To validate the Bloch equation-based forward model, data was acquired on a gel phantom with a T2=60ms with the phase encoding gradients turned off. This allowed extraction of the TSE signal evolution directly from the k-space.
Results
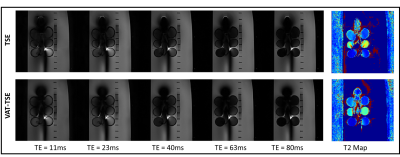
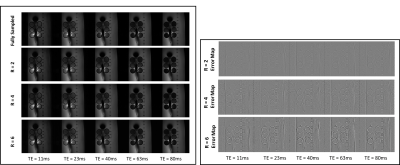
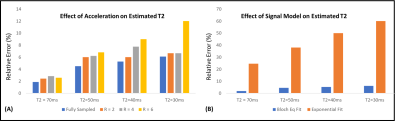
To allow accurate T2 estimation in the presence of stimulated echoes, the low flip angle TSE sequence was simulated using extended phase graph (EPG), slice-resolved EPG (SEPG)[12], and Bloch equations. Figure 2 shows the validity of all three models compared to the acquired signal from a gel phantom. Note the good correspondence between the Bloch equation model and the acquired data for different flip angle combinations.Figure 3 shows the effect of VAT in reducing metal susceptibility artifacts on the T2 maps. Compared to TSE acquisitions, VAT-TSE T2 maps demonstrate more realistic geometry and more homogenous T2 values. The reconstructed TE images from retrospective undersampling are shown in Figure 4 for acceleration factors of R=2,4 and 6. While the error in T2 estimates increases with R = 6, the reconstruction faithfully recovers the image contrast at each TE. R=4 allows TSE-VAT T2 mapping data acquisition in 2.5 min compared to a 10 min fully sampled acquisition. T2 maps were generated from the accelerated data and compared to the reference estimate without the metal implant (Figure 5). Note that with R = 4, the relative error in T2 was less than 6%. Due to the use of low flip angles, using a monoexponential fitting model results in large T2 overestimations (Figure 5B).
Conclusions
We have presented an accurate and efficient model-based method of T2 quantification at the vicinity of metal implants. The proposed method achieves high sampling rates at different TEs while using low flip angles and spatiotemporal undersampling to remain within feasible scan times. We have evaluated the method using simulations and metal phantom experiments. While the utility of this technique is demonstrated on TSE-VAT-based T2 mapping, it can be applied to other metal artifact reduction schemes such as SEMAC.Acknowledgements
No acknowledgement found.References
1. Li X, Majumdar S. Quantitative MRI of articular cartilage and its clinical applications. J Magn Reson Imaging 2013;38:991–1008.
2. David‐Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging 2004;22:673–682.
3. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Seminars Musculoskeletal Radiol 2004;8:355–368.
4. Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 2004; 232:592–598.
5. Bhave S, Koff MF, Sivaram Kaushik S, Potter HG, Koch KM. 3D-multi-spectral T2 mapping near metal implants. Magn Reson Med. 2019 Aug;82(2):614-621. doi: 10.1002/mrm.27744. Epub 2019 Mar 18.
6. Verschueren J, Meuffels DE, Bron EE, Klein S, Kleinrensink GJ, Verhaar JAN, Bierma-Zeinstra SMA, Krestin GP, Wielopolski PA, Reijman M, Oei EHG. Possibility of quantitative T2-mapping MRI of cartilage near metal in high tibial osteotomy: A human cadaver study. J Orthop Res. 2018 Apr;36(4):1206-1212.
7. Cheung J, Neri JP, Gao MA, Lin B, Burge AJ, Potter HG, Koch KM, Koff MF. Clinical Feasibility of Multi-Acquisition Variable-Resonance Image Combination-Based T2 Mapping near Hip Arthroplasty. HSS J. 2021 Jul;17(2):165-173.
8. Jungmann PM, Ganter C, Schaeffeler CJ, Bauer JS, Baum T, Meier R, Nittka M, Pohlig F, Rechl H, von Eisenhart-Rothe R, Rummeny EJ, Woertler K. View-Angle Tilting and Slice-Encoding Metal Artifact Correction for Artifact Reduction in MRI: Experimental Sequence Optimization for Orthopaedic Tumor Endoprostheses and Clinical Application. PLoS One. 2015 Apr 24;10(4):e0124922.
9. Levine E, Daniel B, Vasanawala S, Hargreaves B, Saranathan M. 3D Cartesian MRI with compressed sensing and variable view sharing using complementary poisson-disc sampling. Magn Reson Med. 2017;77(5):1774-1785. doi:10.1002/mrm.26254
10. Keerthivasan MB, Galons JP, Johnson K, et al. Abdominal T2-Weighted Imaging and T2 Mapping Using a Variable Flip Angle Radial Turbo Spin-Echo Technique. J Magn Reson Imaging. 2021 Jul 13. doi: 10.1002/jmri.27825.
11. Ben-Eliezer, N., Sodickson, D.K. and Block, K.T. (2015), Rapid and accurate T2 mapping from multi–spin-echo data using Bloch-simulation-based reconstruction. Magn. Reson. Med., 73: 809-817.
12. Lebel RM, Wilman AH. Transverse relaxometry with stimulated echo compensation. Magn Reson Med. 2010 Oct;64(4):1005-14. doi: 10.1002/mrm.22487. PMID: 20564587.
Figures