3933
Abdominal Water-Only T2 Estimation at 0.55T using a 3D Stack-of-Stars TSE-DIXON Technique1Siemens Medical Solutions USA, New York, NY, United States, 2Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States, 3Department of Radiology, Weill Cornell Medical College, New York, NY, United States, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Siemens Medical Solutions USA, Malvern, PA, United States
Synopsis
We explore the use of a stack-of-stars 3D TSE sequence along with multi-echo DIXON readouts for SNR-efficient, free-breathing water-only T2 estimation at 0.55T. A subspace constrained reconstruction is combined with a multi-step fat/ water separation algorithm to generate water-only T2-weighted images and T2 maps.
Background and Motivation
Abdominal T2-mapping techniques have recently been explored for the quantification of various pathologies including focal liver lesions, renal cell carcinoma, and diffuse pancreatic disease [1-3]. However, presence of fat in the liver acts as a confounding factor, resulting in T2-estimation errors. To overcome the effect of fat, these techniques typically employ spectral fat-suppression schemes [1,2]. Alternative approaches for estimating the water-only T2 component include use of multi-component T2-estimation algorithms [4] and 2D gradient and spin-echo sequences [5].Motivated by the recent interest in <1T low-field imaging [6,7], we explore quantitative abdominal imaging at 0.55T. In this work, we propose the use of a stack-of-stars 3D TSE sequence along with multi-echo DIXON readouts for SNR-efficient, free-breathing water-only T2 estimation. Fat-suppression and T2-estimation performance of the proposed technique is demonstrated using phantoms and in-vivo imaging.
Theory
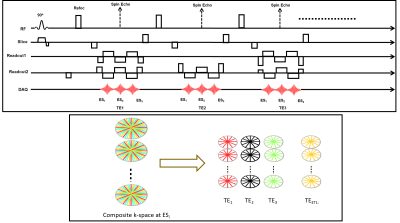
Pulse SequenceWe extended a previously proposed stack-of-stars TSE pulse sequence [8,9] to support multi-echo readouts. Following each refocusing RF pulse, data is acquired at pre-defined echo shifts ($$$ES_i$$$) with respect to the spin echo (Figure 1A). K-space is sampled using a “stack-of-stars” trajectory: Cartesian encoding is applied along the Kz dimension while each partition is sampled using radial readouts. This results in the acquisition of k-space volumes corresponding to each echo shift. The acquired k-space is segmented to generate under-sampled k-space data corresponding to each echo time (TE) and echo shift (ES) (Figure 1B).
Image Reconstruction
The under-sampled k-space data corresponding to the different echo shifts are jointly reconstructed using a subspace-constrained reconstruction algorithm:
$$\hat{M} = arg min_M ||\sum_i^NE_i \Psi M -K_i||_2^2+\lambda R(M)$$ where $$$E=FS$$$ , $$$F$$$ is the Non-Uniform Fast Fourier Transform, $$$S$$$ is the coil sensitivity matrix, $$$\Psi$$$ is a $$$L$$$-dimensional principal component subspace basis such that $$$L<<ETL$$$, and $$$M$$$ are the principal component coefficients for the different echo shifts. The subspace basis $$$\Psi$$$ is generated using a Bloch-equation-based model of the TSE sequence [10], and a locally low rank regularization $$$R$$$ was used.
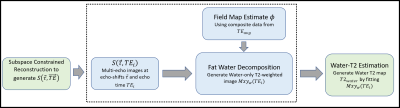
The reconstruction generates images at different TEs and echo shifts. Fat/water separation was performed in a two-step approach (Figure 2): (1) Composite T2w images were generated at each echo shift by combining views from all TEs. These images were used to estimate the background field map > The water-only images from the different TEs were used to estimate a water-only T2 map using Bloch-equation-based fitting.
Methods
Phantom ImagingThe prototype sequence was tested on a commercial MRI system (MAGNETOM Aera 1.5T, Siemens Healthcare, Erlangen, Germany) modified to operate at a field strength of 0.55T. Quantification accuracy was evaluated using a set of emulsions with varying fat fraction and T2. Reference values for fat fraction were obtained using a multi-echo Cartesian GRE sequence, and reference T2 estimates were obtained using a fat-suppressed single echo spin-echo sequence. Data were acquired with the stack-of-stars TSE-DIXON sequence using the following parameters: FOV=26cm, base resolution=256, radial views=400, ETL=40, echo-shifts=[-4,0,4] ms. Acquisition time = 5 min 36 sec.
In-Vivo Imaging
Data were acquired in volunteers after informed consent. Free-breathing abdominal data were acquired using the TSE-Dixon sequence with: FOV=38cm, base resolution=192, radial views=400, ETL=40, echo-shifts = [-4,0,4] ms. Acquisition time = 5 min 36 sec.
Results
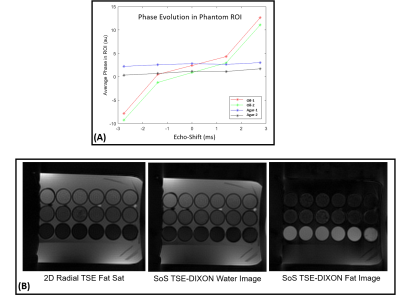
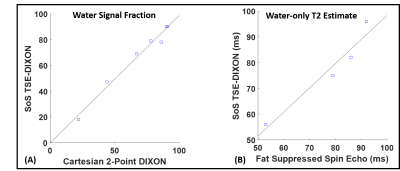
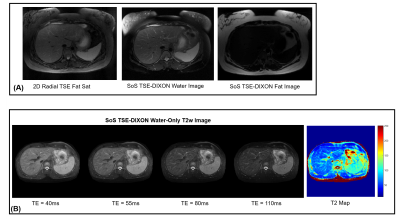
Figure 3A shows the phase evolution within an ROI for a fat-only and water-only phantom. Note that at the spin-echo point (echo shift=0ms), both fat and water are in phase. The proposed technique can generate water-only images with fat-suppression efficiency comparable to spectral fat-saturation acquisition (Figure 3B). Figure 4A shows very good correlation (r=0.9674) in water signal fraction between a conventional 2-point DIXON GRE technique and the proposed 3D TSE-DIXON scheme. Furthermore, the estimated water-only T2 values have good concordance with a reference fat-suppressed spin-echo estimate (Figure 4B).Figure 5A compares the water-only T2w image generated by the stack-of-stars sequence to a 2D radial TSE sequence with SPAIR fat suppression. The proposed technique can generate water-only T2w images at different TE times and a water-only T2 map (Figure 5B).
Conclusions
We present a novel stack-of-stars 3D TSE-DIXON technique for free-breathing water-only T2 estimation. Phantom experiments indicate good agreement with reference and conventional quantification approaches. Preliminary in-vivo results indicate potential applications for quantitative characterization of liver disease.Acknowledgements
No acknowledgement found.References
1. Keerthivasan MB, Galons JP, Johnson K, et al. Abdominal T2-Weighted Imaging and T2 Mapping Using a Variable Flip Angle Radial Turbo Spin-Echo Technique. J Magn Reson Imaging. 2021 Jul 13. doi: 10.1002/jmri.27825.
2. Vietti Violi N, Hilbert T, Bastiaansen JAM, et al. Patient respiratory‐triggered quantitative T 2 mapping in the pancreas. J Magn Reson Imaging. 2019;50(2):410-416. doi:10.1002/jmri.26612
3. Adams LC, Bressem KK, Jurmeister P, et al. Use of quantitative T2 mapping for the assessment of renal cell carcinomas: First results. Cancer Imaging. 2019;19(1):35. doi:10.1186/s40644-019-0222-8
4. Santini F, Deligianni X, Paoletti M, Solazzo F, Weigel M, de Sousa PL, Bieri O, Monforte M, Ricci E, Tasca G, Pichiecchio A, Bergsland N. Fast Open-Source Toolkit for Water T2 Mapping in the Presence of Fat From Multi-Echo Spin-Echo Acquisitions for Muscle MRI. Front Neurol. 2021 Feb 26;12:630387. doi: 10.3389/fneur.2021.630387.
5. Li Z, Graff C, Gmitro AF, et al. Rapid water and lipid imaging with T2 mapping using a radial IDEAL-GRASE technique. Magn Reson Med. 2009 Jun;61(6):1415-24. doi: 10.1002/mrm.21918.
6. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 2019;293(2):384-393.
7. Chandarana, H., Bagga, B., Huang, C. et al. Diagnostic abdominal MR imaging on a prototype low-field 0.55 T scanner operating at two different gradient strengths. Abdom Radiol (2021)
8. Benkert T, Mugler JP 3rd, Rigie DS, et al. Hybrid T2 - and T1 -weighted radial acquisition for free-breathing abdominal examination. Magn Reson Med. 2018;80(5):1935-1948.
9. Keerthivasan MB, Saranathan M, Johnson K, et al. An efficient 3D stack-of-stars turbo spin echo pulse sequence for simultaneous T2-weighted imaging and T2 mapping. Magn Reson Med. 2019 Jul;82(1):326-341.
10. Ben-Eliezer, N., Sodickson, D.K. and Block, K.T. (2015), Rapid and accurate T2 mapping from multi–spin-echo data using Bloch-simulation-based reconstruction. Magn. Reson. Med., 73: 809-817.
11. Hernando D, Kellman P, Haldar J, Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 2010;63:79‐90
12. Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn Reson Med. 2017;78(2):565-576. doi:10.1002/mrm.26392
Figures