3910
A U-Net based Approach to the Prediction of Regions-of-Interest for Metabolic Sodium Imaging1Institut of Neuroscience and Medicine - 4, Forschungszentrum Jülich GmbH, Jülich, Germany, 2Institut of Neuroscience and Medicine - 11, Forschungszentrum Jülich GmbH, Jülich, Germany, 3Department of Neurology, RWTH Aachen University, Aachen, Germany, 4JARA-BRAIN - Translational Medicine, Jülich-Aachen Research Alliance, Aachen, Germany
Synopsis
Sodium MRI yields metabolic information about the brain and might indicate existing or emerging pathologies. Often this information is to be determined in a certain region-of-interest (ROI). These ROIs can be, for example, all grey or white matter regions, or more specific sub-regions thereof and it is important to predict these ROIs without bias. Here, an approach to obtain well segmented ROIs is presented based on a deep neural network architecture.
Introduction
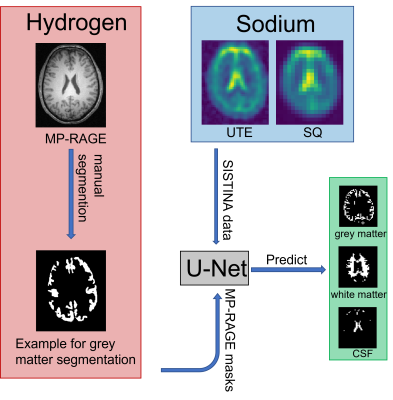
Sodium imaging delivers valuable information about in vivo metabolism, and it enables the measurement of additional parameters regarding the sodium properties of tissue (such as relaxation times and concentrations)1. An enhanced SISTINA sequence2 for sodium imaging with multiple quantum filtering allows the estimation of sodium parameters such as sodium concentrations, volume and molar fractions in given regions-of-interest (ROI). The sequence produces three data sets, the total sodium concentration via the ultra-short echo-time readout (UTE), the single quantum filtered signal (SQ) and the triple quantum filtered signal (TQ). The combination of these measurements as well as accurate segmentations enable the evaluation and estimation of sodium parameters.Methods
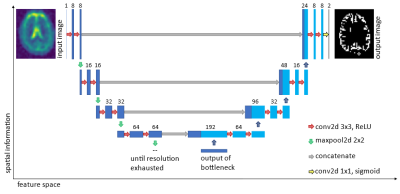
A U-Net-based model is used to learn the interdependencies between sodium weighted images and proton weighted images. To decrease the overfitting properties of the network, data augmentation methods3 have been applied to artificially increase the training data set. The extraction of information was performed on measurements of 36 healthy subjects using a 4T scanner1. The ground truth of the segmentation is based on the manual segmentation of proton images using an MP-RAGE sequence recorded in the same session as the enhanced SISTINA sequence. The MP-RAGE images are co-registered to an MNI atlas4 and based on the co-registration, the ROIs are segmented for the hydrogen images. These masks are then downsampled to the resolution of the UTE or SQ/TQ data sets respectively and are thereafter used for the training of the U-net (see Figure 1). The output of the U-Net is evaluated using the Rand index5. The Rand index is quantifies the similarity between two distributions (in this case the segmentation maps). A perfect match would lead to a Rand index of 1, whereas two disjunct distributions would yield a Rand index of 0.Results
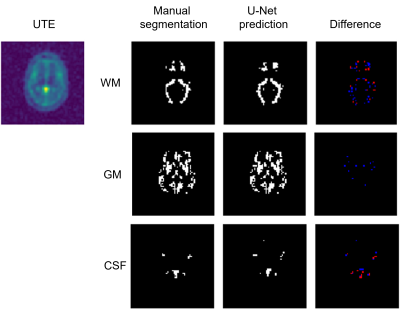
It can be shown via the network that the features of the brain structure are also contained in the sodium images. The performance varies depending on the data set and the applied mask but, nevertheless, in general the major anatomical structures in the brain can be reconstructed using only the sodium information. Figure 3 shows an example of the performance of the U-net for determining the ROI containing white matter, grey matter and cerebrospinal fluid.Conclusion
Once the neural network is sufficiently trained, it is able to yield unbiased predictions of the ROIs without using the data from the MP-RAGE sequence. It is well possible that additional acquisitions for the sole purpose of segmenting metabolic images can be avoided and thus yielding a reduction in scan time for the patient. Segmentation is a tedious, time consuming task, usually performed by radiologists. An automatic procedure without the need for any human interaction is a desirable prospect. A machine learning approach is elegant, since there is no need for an explicit expression of anatomical features within the brain.Acknowledgements
The authors thank Dr. Aliaksandra Shymanskaya for her assistance with the measurements.References
1. Worthoff et al. Relaxometry and quantification in simultaneously acquired single and triple quantum filtered sodium MRI. Magn Reson Med. 2019;81:303-315.
2. Fiege et al. Simultaneous Single-Quantum and Triple-Quantum-Filtered MRI of 23Na (SISTINA). Magn Reson Med. 2013;69(6):1691-1696.
3. Ronneberger et al. U-Net: Convolutional Networks for Biomedical Image Segmentations MICCAI. 2015;234-241.
4. Chau et al. The Talairach coordinate of a point in the MNI space: how to interpret it. Neuroimage . 2005;25(2):408-16.
5. Buslaev et al. Albumentations: fast and flexible image augmentations Inf. 2020;11(2),125.
6. Unnikrishnan et al. Measure of Similarity. Proc. IEEE Wortkshop Computer Vision Applications. 2005;1:394-394
Figures