3907
Brain SMS-EPI reconstructions with prospective motion correction: improved quality with updated coil sensitivity maps1Diagnostic Radiology and Nuclear Medicine, University of Maryland, Baltimore, Baltimore, MD, United States, 2U.S. Food Drug Administration, Silver Spring, MD, United States, 3Department of Mathematics, University of Maryland, College Park, College Park, MD, United States
Synopsis
We investigated prospective motion correction (PMC) for brain SMS-EPI using camera-based motion tracking and coil sensitivity interpolation. Phase compensation for the receiver was performed to eliminate motion-induced aliasing artifacts. Coil sensitivity profiles were interpolated and extrapolated with Makima piecewise cubic Hermite algorithm to remove residual SMS reconstruction artifacts caused by the misregistration of coil sensitivities between updated (mobile) SMS slices and stationary single-slice reference profiles.
Introduction
SMS-EPI reduces volume acquisition times for functional and diffusion MRI (1), but long scan times are commonly needed for both fMRI (2) and diffusion MRI (3). Motion-induced errors in RF coil sensitivity profiles and the phase of numerically controlled oscillator (NCO) can induce aliasing artifacts, even when prospective motion correction (PMC) is performed. We previously proposed a technique to eliminate these aliasing artifacts in SMS-EPI (4), but it was only assessed in phantoms. Here we report on initial in vivo results.Materials and Methods
The study was performed on a 3 T whole-body MRI scanner (Prisma, VE13C, Siemens Healthcare, Germany) equipped with a 20-channel head coil. A gradient-echo SMS-EPI sequence was modified to continuously receive and update motion parameters in real-time. A camera system was used to track the movement of a marker (accuracy 0.1mm and 0.1°; 60Hz frame rate). NCO real-time phase compensation was performed for each k-space line to eliminate motion-induced aliasing artifacts (4). Split slice-GRAPPA (SP-SG) (5) was used to unfold the SMS images. The weight to synthesize data of channel $$$j$$$ at location $$$z\in\left[1...NS\right]$$$ is $$W_z^j=\left(\sum_{s=1}^{NS}\left(K_s^*K_s\right)\right)^{-1}K_z^*T_z^j,[1]$$ where $$$NS$$$ is the number of slices, and $$$K_1...K_s$$$ are convolution matrices across channels determined from single-slice prescan data. $$$T_z^j$$$ is the $$$jth$$$ channel data at location z.In the presence of motion, for a particular point in time $$$t$$$ and with camera-based PMC enabled, the signal $$$m$$$ at point number $$$l$$$ in k-space of the $$$jth$$$ channel is given by $$m_{l,j,t}=\int\rho\left(r\right)e^{-ik_lr}C_{j,t}\left(r\right)dr,[2]$$ where $$$\rho$$$ is spin density, r denotes position, and C is the coil sensitivity map. r is invariant to time, and $$$k_l$$$ has no change since PMC is enabled. However, since coils are stationary, they show apparent motion relative to the mobile brain reference frame and C changes with time. Therefore, with PMC enabled, if coil sensitivity profiles are not updated with motion, SMS reconstruction can show artifacts because of the mismatch in the coil sensitivity encodings between SMS collapsed data and the reference data. However, the artifacts can be eliminated by recalculating $$$K$$$ and $$$T$$$ and eventually GRAPPA weights using updated coil sensitivity profiles. We used motion parameters to update coil sensitivity profiles by complex interpolation and extrapolation with the Makima piecewise cubic Hermite method (6).
Three male volunteers (age 35.3±5.9 years) were enrolled, and written informed consent was received from each subject as approved by the local IRB. For each subject, three SMS-EPI sequences were acquired with 2, 3 and 4-fold SMS acceleration. PMC was enabled, and real-time phase compensation of the NCO was turned on for each scan. The scan parameters were TR/TE=3000/30ms, 20 axial slices of 5mm thickness, slice center-to-center gap 10mm, FOV 240 × 240mm2, matrix size 84 × 84, 80 time points. The total scan time was about 50 minutes. The initial volumes of each scan were collected without subject motion. Data were reconstructed offline using SP-SG.
Results
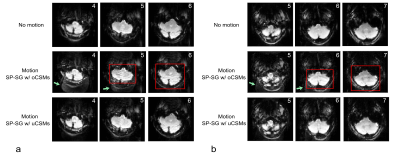
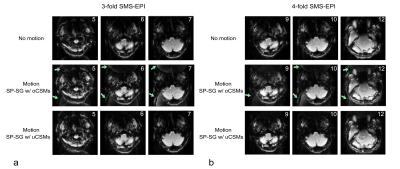
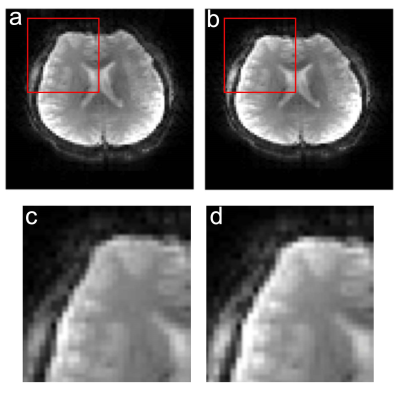
Reconstructions of SP-SG scans from 2 subjects with 2-fold acceleration are shown in Figure 1, using original coil sensitivity maps (oCSMs) and updated coil sensitivity maps (uCSMs). Movements were primarily z-rotations (Fig.1a: -18.7° plus 5.6mm x-translation; Fig.1b: 9.5° plus y-translation 7.2 mm). Reconstructions with oCSMs cause residual aliasing artifacts (middle row, green arrows) as well as ripple artifacts in the cerebellum (red boxes). These reconstruction artifacts disappear when uCSMs are used for reconstruction (Fig.1 bottom row).Figure 2 shows results with 3-fold and 4-fold SMS-EPI accelerations. Again, reconstructions using oCSMs show residual aliasing artifacts for both accelerations (green arrows, middle row), which are eliminated in the images with uCSMs (bottom row). Figure 3 shows additional 2-fold SMS-EPI reconstructions with oCSMs and uCSMs. The gray-white matter contrast is improved in images reconstructed with uCSMs (Fig. 3d, zoomed view) compared to those with oCSMs (Fig. 3c zoomed view). This indicates that SMS reconstructions without updated CSMs can cause signal errors.
Discussion
We found that interpolation and extrapolation of CSMs with Makima is superior to those using Spline and Pchip interpolations, most likely since Makima, as a low-order polynomial interpolation, is less prone to overshoot and undershoot when the data are constant for more than two consecutive nodes. Therefore, Makima interpolations tend to be smooth and are well-suited for the continuity and smoothness requirements of coil sensitivity maps.Split slice-GRAPPA acquisitions with PMC reconstructed with original CSMs may show artifacts when motion occurs, but reconstruction with uCSMs can significantly reduce these artifacts and provide high-quality SMS-EPI images. In phantom experiments, we found noticeable artifacts for translations ≥10mm or rotations ≥5°; motion amplitudes that were exceeded in our human studies. Of note, while our implementation used an optical system for motion tracking, it is likely that MR-based motion correction techniques, such as PACE (7), would show similar artifacts for larger movements.
Acknowledgements
This work was supported by NIH grant 1R01 DA021146 (BRP) and NSF grants DMS-1816608 and DMS-2108900. We also would like to acknowledge Drs. Pan Su and Tobias Kober from Siemens Healthineers for providing technical assistance and expertise.References
1. Barth M, Breuer F, Koopmans PJ, Norris DG, Poser BA. Simultaneous multislice (SMS) imaging techniques. Magn Reson Med 2016;75(1):63-81.
2. Sanchez-Panchuelo RM, Besle J, Beckett A, Bowtell R, Schluppeck D, Francis S. Within-digit functional parcellation of Brodmann areas of the human primary somatosensory cortex using functional magnetic resonance imaging at 7 tesla. J Neurosci 2012;32(45):15815-15822.
3. Herbst M, Poser BA, Singh A, Deng W, Knowles B, Zaitsev M, Stenger VA, Ernst T. Motion correction for diffusion weighted SMS imaging. Magn Reson Imaging 2017;38:33-38.
4. Bo Li, Ningzhi Li, Ze Wang, Thomas Ernst. SMS-EPI real-time motion correction by receiver phase compensation and coil sensitivity interpolation. Proceedings of the 28th Annual Meeting of ISMRM; 2007. p. 1369.
5. Cauley SF, Polimeni JR, Bhat H, Wald LL, Setsompop K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn Reson Med 2014;72(1):93-102.
6. https://www.mathworks.com/help/matlab/ref/makima.html.
7. Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457-465.
Figures