3905
NOise Reduction with Distribution Corrected (NORDIC) PCA improves signal-to-noise and functional connectivity in rodent resting-state fMRI1Department of Ophthalmology, New York University Grossman School of Medicine, New York, NY, United States, 2Neuroscience Institute, New York University Grossman School of Medicine, New York, NY, United States, 3Center for Magnetic Resonance Research (CMRR), University of Minnesota, Minneapolis, MN, United States, 4Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States
Synopsis
The relatively poor temporal signal-to-noise ratio (tSNR) in resting-state fMRI (rsfMRI) serves to be a pressing area of improvement. NOise Reduction with Distributed Corrected (NORDIC) PCA can effectively increase tSNR. However, it has yet to be examined in rodent rsfMRI studies. Here, we applied NORDIC-correction to mouse rsfMRI, and evaluated the tSNR and functional connectivity against standard preprocessing data. NORDIC was able to significantly increase tSNR and reduce functional connectivity variability. NORDIC can also denoise rsfMRI signals at higher frequencies. Taken together, NORDIC can potentially become an important preprocessing step in future rodent rsfMRI studies.
Introduction
Resting-state fMRI (rsfMRI) serves as a baseline function of spontaneous activity and provides insights into brain functional networks1,2. However, rsfMRI suffers from relatively poor temporal signal-to-noise ratio (tSNR), caused by a multitude of factors including thermal noise, making this a pressing area of improvement. Thermal noise here is defined by a zero-mean Gaussian distributed noise generated by the MRI machines itself and/or the body temperature of the subject3. Thermal noise itself can be affected by factors, including voxel volume and/or magnetic field strengths, making it a significant part of rsfMRI acquisition4,5. Current denoising techniques such as NOise Reduction with Distribution Corrected (NORDIC) PCA have focused on human MRI6,7, while this correction has not yet been examined in rodent MRI11. Due to the high translational value of rodent to human rsfMRI, understanding the effect of NORDIC on rodent rsfMRI can facilitate better human rsfMRI study design and data analysis. Here, we applied NORDIC with standard rodent pre-processed rsfMRI to evaluate if the improved tSNR also improves rsfMRI indicators such as functional connectivity and power spectrum changes.Methods
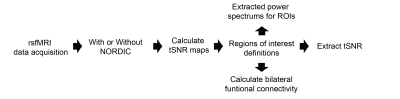
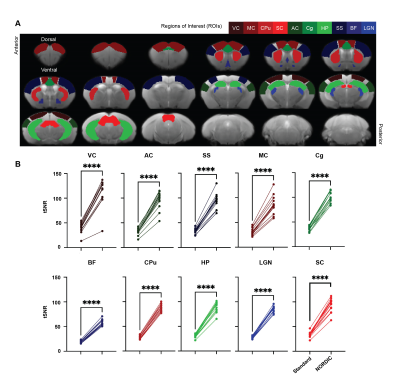
rsfMRI images were obtained from 13 adult male C57BL/6J mice using a 7-Tesla Bruker scanner and single-shot echo-planar-imaging (EPI) pulse sequence with TE/TR=12/1000ms, FOV=16x7mm2, 80x35 matrix, 30 contiguous 0.5-mm axial slices, and 600 volumes, magnitude, and phase data. NORDIC12 and standard preprocessing were applied, including slice timing correction, realignment, spatial smoothing, and temporal low-pass filtering. tSNR maps were then calculated by dividing voxel-wise mean by voxel-wise standard deviation. Regions of interest (ROIs) were defined across cortical and subcortical regions to assess tSNR, functional connectivity, and power spectra (Figure 1). Functional connectivity was calculated by obtaining the correlation coefficients between the ROIs in the left and right hemispheres. Paired t-tests, Levene’s F-test, and 2-way ANOVA were implemented for statistical analysis.Results
NORDIC PCA increased tSNR in rodent rsfMRI
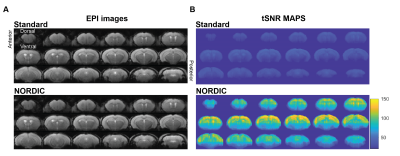
Our initial phenotypic analysis of the rodent rsfMRI EPI images showed no apparent morphological changes with and without NORDIC-correction (Figure 2A). NORDIC-corrected rsfMRI data had higher tSNR qualitatively compared to the standard preprocessed data (Figure 2B). After extracting the tSNR values from the maps, we found a significant interaction effect between ROI and NORDIC-correction, F(2.753, 33.039)=15.281, p=0.000003, ηp2=0.560. To explore this effect further, we conducted a series of planned-comparisons between standard and NORDIC-corrected data for each ROI, and found that all comparisons were significant (p<0.000001). This indicates that NORDIC-correction increased tSNR for all ROIs and that NORDIC-correction was successful in denoising and increasing the signal (Figure 3).Functional Connectivity variability was reduced by NORDIC
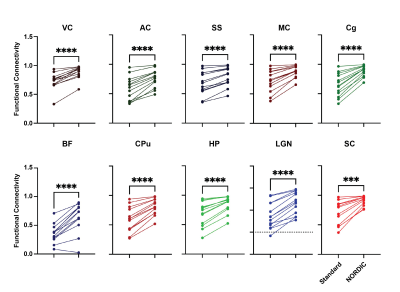
We found a significant interaction effect between ROI and NORDIC-correction, F(2.621, 31.448)=7.193, p=0.001252, ηp2=0.375, while Levene’s F-test showed that variability within the functional connectivity data decreased after NORDIC-correction was applied (Figure 4). To explore this effect further, we conducted a series of planned-comparisons between standard and NORDIC-corrected data for each of the ROIs. Here, we found that all comparisons were significant (all p<0.0005). This indicates that NORDIC-corrections also increased the bilateral functional connectivity for all ROIs (Figure 4).NORDIC suppresses higher frequency noise in small ROIs
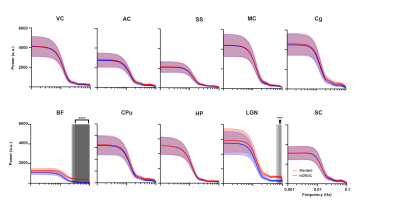
Only the basal forebrain (BF) and lateral geniculate nucleus (LGN) had a significant difference between the standard preprocessed and NORDIC-corrected data within the relatively higher frequencies of the power spectrum (0.024-0.1Hz, and 0.067-0.1Hz, respectively; Figure 5). BF and LGN are both of smaller volumes than other ROIs measured, thus making them more susceptible to noise and, subsequently, corrected by NORDIC PCA. Since lower frequencies mainly contribute to rsfMRI signals8, this suggests that NORDIC-correction successfully suppressed higher frequency noise in BF and LGN and did not affect the rsfMRI signals.Discussion
Since NORDIC-correction was effective in denoising and increasing the signal in rsfMRI, this possibly will allow for more assessments of reliable brain functional networks. The implications of this could allow better detection of changes in inter-regional correlation between varying brain regions, such as visual, motor, and somatosensory cortices, in future studies, as NORDIC effectively decreases variability in functional connectivity in these regions. Since smaller ROIs (BF and LGN), showed a suppression of higher frequency noise with NORDIC, future studies segmenting different cortical layers, as well as functional brain subregions, are warranted, potentially shedding light on rodent laminar and columnar fMRI. Rodent diffusion MRI can be another area of future NORDIC-correction, as it is crucial in delineating finer brain structures and connectomics, which suffers similarly poor tSNR6,9. Future studies may also encompass the effects of NORDIC PCA on tasked-based rodent fMRI. Furthermore, optogenetic fMRI8,10 (ofMRI) has enabled to directly probe the causal influence of genetically defined neurons on downstream regions with whole-brain coverage. However, most ofMRI studies utilize a looped room temperature surface coil which suffers more thermal noise compared to the cryo-probe which is used in typical rodent fMRI studies. Incorporating NORDIC-correction in the preprocessing pipeline may drastically improve future ofMRI studies.Conclusion
NORDIC-correction significantly increased tSNR and reduced functional connectivity variability, allowing for a potentially more robust analysis of spontaneous brain activity and functional connectivity. Higher frequencies of the power spectrum seem to be primarily suppressed in the BF and LGN, indicating that NORDIC-correction was effective for denoising small brain volumes. Future studies are warranted that apply these methods to experimental disease models to further evaluate the potential of NORDIC-correction for preclinical rsfMRI research.Acknowledgements
This work was supported in part by the National Institutes of Health P30-CA016087, P41-EB017183, R01-EY028125, P41-EB027061, U01 EB025144 and UF1-NS107680 (Bethesda, Maryland); BrightFocus Foundation G2019103 (Clarksburg, Maryland); and an unrestricted grant from Research to Prevent Blindness to NYU Langone Health Department of Ophthalmology (New York, New York).References
1 Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34, 537-541, doi:10.1002/mrm.1910340409 (1995).
2 Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8, 700-711, doi:10.1038/nrn2201 (2007).
3 Brooks, J. C., Faull, O. K., Pattinson, K. T. & Jenkinson, M. Physiological noise in brainstem FMRI. Front Hum Neurosci 7, 623, doi:10.3389/fnhum.2013.00623 (2013).
4 de Zwart, J. A., Gelderen, P., Fukunaga, M. & Duyn, J. H. Reducing correlated noise in fMRI data. Magn Reson Med 59, 939-945, doi:10.1002/mrm.21507 (2008).
5 Triantafyllou, C., Polimeni, J. R. & Wald, L. L. Physiological noise and signal-to-noise ratio in fMRI with multi-channel array coils. Neuroimage 55, 597-606, doi:10.1016/j.neuroimage.2010.11.084 (2011).
6 Moeller, S. et al. NOise reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing. Neuroimage 226, 117539, doi:10.1016/j.neuroimage.2020.117539 (2021).
7 Vizioli, L. et al. Lowering the thermal noise barrier in functional brain mapping with magnetic resonance imaging. Nat Commun 12, 5181, doi:10.1038/s41467-021-25431-8 (2021).
8 Chan, R. W. et al. Low-frequency hippocampal-cortical activity drives brain-wide resting-state functional MRI connectivity. Proc Natl Acad Sci U S A 114, E6972-E6981, doi:10.1073/pnas.1703309114 (2017).
9 Chen, B. & Hsu, E. W. Noise removal in magnetic resonance diffusion tensor imaging. Magn Reson Med 54, 393-401, doi:10.1002/mrm.20582 (2005).
10 Lee, J. H. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792, doi:10.1038/nature09108 (2010).
11 Cho, Shinho et al. High Resolution Functional Mapping of Orientation Domains in the Cat Visual Cortex using Denoising with NORDIC. ISMRM 2916, (2021)
12 SteenMoeller. “Steenmoeller/nordic_raw: MATLAB Code for Performing Image Reconstruction in MRI and Performing the Nordic Denoising.” GitHub, https://github.com/SteenMoeller/NORDIC_Raw.
Figures