3904
Spatial alignment of structural images on common and RESOLVE diffusion images in the optic nerve1Neuroradiology, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, 2Applied Physics & Medical Engineering, Hochschule RheinMain, Rüsselsheim, Germany, 3Ophthalmology, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
Synopsis
A successful use of anatomical templates in the evaluation of functional neuroimaging-studies is only possible, if anatomical and functional intra-individual data can be registered with sufficient accuracy. We wanted to investigate the possibility to register structural on common and RESOLVE diffusion-images of the optic nerve. Three volunteers were examined and the structural images were registered to the two diffusion-datasets. Two observers defined seeds for tractography of the optic nerve on the structural and on the diffusion images. Tractography worked with seeds defined on the structural images registered to RESOLVE. Therefore RESOLVE is suitable for the use in template-based studies.
Introduction
The evaluation of neuroimaging data of the brain is often based on the use of standardized templates to save time, make results more objective or do group analyses. It is especially helpful when evaluating functional or diffusion data because of their low resolution and image quality. Templates enable the use of predefined seeds [1] or whole tracks-regions [2] for tractography. Unfortunately the common templates do not include the optic nerve. Published experiences of matching the optic nerve to a template do not exist to our knowledge.One step prior to template registration, a registration between structural and functional data has to take place. This is a crucial step that influences the whole outcome of the registration process. In preparation to the development and the use of a template including the optic nerve, we wanted to determine, whether the prepended step of registering structural images to often distorted diffusion data is possible. We compare our approach for a common diffusion weighted sequence and for a readout-segmented, multi-shot echo planar imaging ((EPI) RESOLVE), which has shown to be less sensitive to distortion artefacts in brain imaging [3,4].
Methods
Imaging was performed on a 3.0 T MR-scanner (Magnetom Skyra, Siemens, Germany) using a 64-channel head-coil. Three healthy volunteers underwent diffusion-MRI using a common and a RESOLVE pulse-sequence covering the optic tract. (2.5mm³ isometric voxel, b=800 and 1600 s/mm², 30 directions, 16 slices). For the common EPI-based diffusion pulse-sequence this resulted in an acquisition-time of 3:02 min and for RESOLVE (R-factor = 3) in 10:27 min. Moreover a structural dataset was acquired in the same position and orientation with higher resolution (3D volumetric interpolated breath-hold sequence (VIBE), voxel size 1x0.8x0.8 mm³, TR 6.42 ms, TE 3.69 ms).All datasets were preprocessed using an in-house rebuild of the tractoflow pipeline (intended for global brain diffusion preprocessing) for use on defined slabs, including the bulbi, the osseous orbita and the intracranial part of the optic nerve. ([5], MRTRIX3 [6], ANTS [7], FSL [8] and scilpy (Sherbrooke Connectivity Imaging Lab) including correction for distortions). For registration of the structural images to the respective diffusion images a 6-parameter rigid body transformation was used (FSL FLIRT [9] via MRTRIX3).
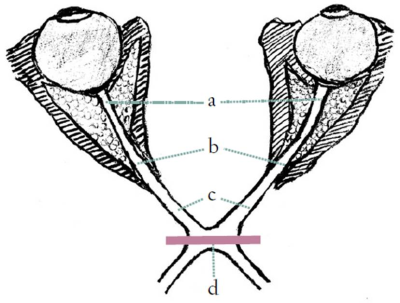
Two experienced neuro-radiologists placed regions of interest (ROI) in a scheme taken from Haykal et al [10] (Fig. 1) in each registered structural image as well as in the two diffusion datasets.
Tracts were generated via MRTRIX3 using the probabilistic iFOD2 algorithm. The ROI closest to the bulbus served as seeding points and all tracts had to include the other two ROI on each side, concluding in the chiasma. Tractography parameters were fixed according to our preliminary experiments in tracking the optic nerve (max angle 15, diameter of sphere-ROI 2.5mm, unidirectional), while the cutoff-value varied over three iterations of the experiment (0.05, 0.075, 0.1). This was inspired by Takemuras approach of ensemble tractography [11] and utilized to get an early pre-metric insight into connection/tract quality. A tract-goal was set for 4000 selected streamlines, the number of seeding points was limited to 2 million. Tracking was counted as successful if the algorithm selected more than 50 tracks. All Tractograms were inspected and checked for plausibility.
Results
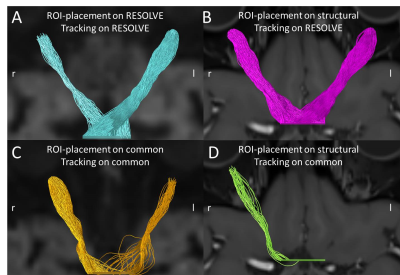
The registration of spatial and diffusion images successfully finished in all cases. The number of successful tractographies can be received from Tab. 1. Examples for successful and (partial) unsuccessful tractrographies are shown in Fig. 2.The feedback of ROI-placement by the neuroradiologists was very positive for all structural volumes, while in both DWI images, especially the intracranial ROI, as well as the chiasma-ROI were described as extremely challenging up to mere “guesswork”.
Results for all cutoff-values were consistent: the highest success-rate was recorded for ROI drawn on structural RESOLVE-registered images followed by those on the RESOLVE-images. Structurally-drawn common-registered ROI however did slightly worse than those drawn directly on the common diffusion-weighted images.
Discussion
Fig.2 illustrates the neuroradiologist's feedback and combines it with supposed underlying reasons for our results. Fig.2A/ B are both based on RESOLVE-data and showed near perfect tracking results, given the provided parameters. This validates a combined success of the preprocessing steps on the RESOLVE-data which in turn facilitated a successful registration. On the other hand, the distortion correction on the common diffusion-weighted images, especially the intracranial segment and around the chiasma, was less successful as can be seen in Fig.2C by the deviating course of the tracks, compared to the structural information. This in turn doomed the common-registered positioning to fail. The ability of the ROI drawn on the structural data to serve as seed-points for tracking of RESOLVE-data with comparable success to the ROI drawn on the RESOLVE-data itself proves, that the preprocessing steps and the registration of structural and diffusion data of the optic nerve is possible for RESOLVE but not for common diffusion-imaging techniques.Eventually, the use of a 12-parameter affine transformation would have led to better results for the common diffusion data, even if it is not usual to use a 12-parameter affine transformation for intra-subject registration.
Conclusion
RESOLVE is a suitable registration target for structural images. Template-development seems to be viable and will be the next focus on our project's agenda.Acknowledgements
No acknowledgement found.References
1. Li, M., et al., The trajectory of the medial longitudinal fasciculus in the human brain: A diffusion imaging-based tractography study. Hum Brain Mapp, 2021.
2. Archer, D.B., D.E. Vaillancourt, and S.A. Coombes, A Template and Probabilistic Atlas of the Human Sensorimotor Tracts using Diffusion MRI. Cereb Cortex, 2018. 28(5): p. 1685-1699.
3. Porter, D.A. and R.M. Heidemann, High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med, 2009. 62(2): p. 468-75.
4. Koyasu, S. et al., The clinical utility of
reduced-distortion readout-segmented echo-planar imaging in the head and
neck region: initial experience.
Eur Radiol, 2014. 24, 3088–3096.
5. Theaud, G., et al., TractoFlow: A robust, efficient and reproducible diffusion MRI pipeline leveraging Nextflow & Singularity. Neuroimage, 2020. 218: p. 116889.
6. Tournier, J.D., et al., MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage, 2019. 202: p. 116137.
7. Avants, B.B., et al., A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 2011. 54(3): p. 2033-44.
8. Jenkinson, M., et al., Fsl. Neuroimage, 2012. 62(2): p. 782-90.
9. Jenkinson, M., et al., Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 2002. 17(2): p. 825-41.
10. Haykal, S., N.M. Jansonius, and F.W. Cornelissen, Investigating changes in axonal density and morphology of glaucomatous optic nerves using fixel-based analysis. Eur J Radiol, 2020. 133: p. 109356.
11. Takemura, H., et al., Ensemble Tractography. PLoS Comput Biol, 2016. 12(2): p. e1004692.
Figures