3893
Neural excitability through BOLD variability in the preterm brain: A longitudinal resting-state fMRI study
Serafeim Loukas1,2, Joana Sa de Almeida1, Djalel Eddine Meskaldji1,3, Dimitri Van De Ville2,4, and Petra Susan Hüppi1
1Division of Development and Growth, Department of Pediatrics, University of Geneva, Geneva, Switzerland, 2Institute of Bioengineering, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Institute of Mathematics, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Department of Radiology and Medical Informatics, University of Geneva, Geneva, Switzerland
1Division of Development and Growth, Department of Pediatrics, University of Geneva, Geneva, Switzerland, 2Institute of Bioengineering, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Institute of Mathematics, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Department of Radiology and Medical Informatics, University of Geneva, Geneva, Switzerland
Synopsis
Resting-state functional connectivity based on simultaneous BOLD oscillations has been described both in preterm infants and fullterm newborns. During this period of rapid cerebral cortex development, different brain activity patterns (networks) have been previously described. Regional changes in spontaneous BOLD signal variability on the other hand reflect local intravoxel BOLD changes related to neural excitability/flexibility. In the present study, we aimed to explore the longitudinal evolution of the BOLD variability between birth and term-equivalent-age (TEA), a period of rapid brain development, in preterm-born infants. Our findings suggest increased variability at TEA as compared to birth in important primary cortices.
Introduction
Resting-state functional MRI (rs-fMRI) reveals spontaneous fluctuations of the BOLD signal, which depends upon cerebral blood flow, volume, and oxygenation1. During early brain development, it has been shown that preterm infants’ brain expresses regional and age-specific rs-fMRI activity patterns2. Given that the intrinsic fluctuations measured by rs-fMRI are a consequence of oxygenated blood supplying active neurons, the BOLD signal variability (BOLD SD) may serve as a physiological signal related to an individual’s cerebrovascular status3 and neural flexibility/excitability4. We hypothesized that rs-BOLD variability during early development may reflect neuronal and cerebrovascular maturity (optimal brain function5). To this end, we aimed to investigate, for the first time, changes in BOLD variability across two time-points in the developing preterm infant brain.Methods
Cohort: Twenty-two (n=22) preterm infants were recruited at Geneva University Hospital neonatal units. All infants underwent magnetic resonance imaging at 33 weeks (mean±SD: 29.23±2.31, range: 24.57-32.57 and at term-equivalent-age (TEA; 40 post-conception weeks; PCWs) Thus, the neuroimaging dataset consists of longitudinal imaging scans.MRI acquisition: An EPI sequence (TR=700ms, Siemens 3T-MAGNETOM) was used resulting in 590 volumes/subject/time-point. No sedation was used and the infants were scanned while resting quietly in the scanner with MR-compatible earmuffs.
Preprocessing: For each subject and time-point, the functional data were realigned and co-registered using SPM12. Volumes with a frame-wise displacement (FD>0.5mm) were removed, along with the previous and two subsequent images. Nine subjects with more than 1/3 of motion-affected volumes were excluded. The UNC neonatal atlas6 was registered to each subject’s native space using Advanced-Normalization-Tools7. The deformation field was applied to the atlas Gray/White/CSF tissues (TMPs) and to the atlas image to bring them into the native space. Using these TPMs, the subject’s T2 is segmented and the subject-specific probability maps were obtained. GM voxel signals were extracted by masking the atlas using the GM TPM obtained by the segmentation, and by reslicing it to the functional space. The regions of the UNC atlas were further grouped into 11 primary regions for a more global brain coverage: Orbito-frontal cortex (OFC), Dorsolateral prefrontal cortex (DFC), Ventrolateral prefrontal cortex (VFC), Medial prefrontal cortex (MFC), Primary motor cortex (M1C), Primary somatosensory cortex (S1C), Posterior inferior parietal cortex (IPC), Primary auditory cortex (A1C), Posterior superior temporal cortex (STC), Inferior temporal cortex (ITC), Primary visual cortex (V1C). Average regional BOLD time-courses were extracted for each subject and finally, the BOLD variability (BOLD SD) was estimated as the standard deviation of the signal change across time, after bandpass filtering [0.01Hz-0.1Hz] (Figure 1).
Analysis: For each subject and time-point, we extracted the BOLD SD measures and we compared them across time-points (33 vs 40 weeks). Paired statistical tests were used to assess the statistical significance of our results.
Results
Regional and temporal differences in the evolution of the BOLD variability (SD) were found between 33 and 40 weeks time-points for the preterm infants. When comparing the BOLD variability evolution across the two time-point, the regions exhibiting the highest increase in BOLD SD (delta: TEA > birth) were found to be the primary visual (V1C), primary somatosensory (S1C), primary auditory (A1C), primary motor (M1C) and posterior inferior parietal cortex (Figure 2 & 4). These results were significant after applying FDR correction for multiple comparisons (α=0.05) (Figure 4). On the other hand, in the frontal and temporal brain areas such as the orbito-frontal cortex (OFC), dorsolateral prefrontal cortex (DFC), ventrolateral prefrontal cortex (VFC), medial prefrontal cortex (MFC), and inferior temporal cortex (ITC), the BOLD variability was not significantly increased or decreased (Figure 3 & 4).Discussion & Conclusion
In the present study, we explored the longitudinal evolution of the BOLD variability between birth and TEA, a time window of rapid brain development. Previous research has shown that the brain is a variable, dynamic system that fluctuates naturally from moment-to-moment4. Moreover, it has been reported that the BOLD variability of neuronal responses may reflect neural flexibility and maturity5. In particular, increased variability in neural systems is reported to be reflective of functional maturation including flexibility, adaptability, and higher dynamic range4.Our resting-state BOLD variability results highlight the increasing role of the primary visual, somatosensory, and motor cortices during this early extrauterine development of preterm infants. The BOLD SD was significantly increased in V1C, S1C, A1C, which are primary sensory areas, as well as in M1C, the primary motor cortex, and the IPC, which is known to be implicated in the integration of sensory information. These findings indicate that major hemodynamic changes occur in these brain regions between birth (33 weeks) and term-equivalent-age (40 weeks), providing evidence in favor of the rapid and early functional (linked to neuronal flexibility) and cerebrovascular maturity of the primary sensory and motor cortices during early development. However, these two are hard to disentangle. On the other hand, the lack of BOLD SD change in the higher-order cortical regions (i.e., frontal/prefrontal areas) suggests that these regions remain immature in terms of functional maturation. This is reasonable given that the frontal regions are late-developing regions of the neocortex8. BOLD variability could, therefore, be a valuable and meaningful biomarker of functional neuronal maturity.
Acknowledgements
This study was supported by the Swiss National Science Foundation n°32473B_135817/1 and the foundation Prim’ enfance. We thank the Plateforme de Recherche de Pédiatrie and the Centre for Biomedical Imaging (CIBM) of the University Hospital of Geneva for their support.References
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage 2004; 23: S220-S33.
- Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 2010;20: 2852-2862.
- Makedonov I., Black S. E., and MacIntosh B. J. BOLD fMRI in the white matter as a marker of aging and small vessel disease. PLoS One 2013; 8:e67652.
- Garrett DD, Samanez-Larkin GR, MacDonald SWS, Lindenberger U, McIntosh AR, Grady CL, 2013. Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neurosci. Biobehav. Rev. 37, 610–624.
- Nomi JS, Bolt TS, Chiemeka Ezie CE, Uddin LQ, Heller AS, 2017. Moment-to-moment BOLD signal Shi F, Yap PT, Wu G, et al. Infant Brain Atlases from Neonates to 1- and 2-year-olds, PLOS One 2011;6(4).
- Shi F, Yap PT, Wu G, et al. Infant Brain Atlases from Neonates to 1- and 2-year-olds, PLOS One 2011;6(4).
- Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration, NeuroImage 2011;54(3).
- Fuster JM, 2002. Frontal lobe and cognitive development. J. Neurocytol. 31, 373–385
Figures
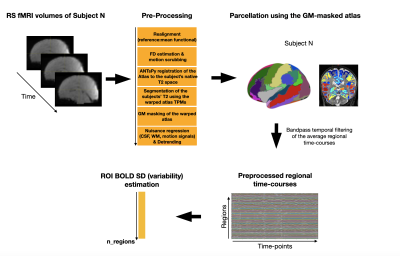
Figure 1: Flowchart showing the preprocessing pipeline and BOLD variability
estimation. This procedure was repeated for each subject and time-point. Following
the preprocessing of the data, the gray matter (GM) mask was used to extract only
GM BOLD signals.
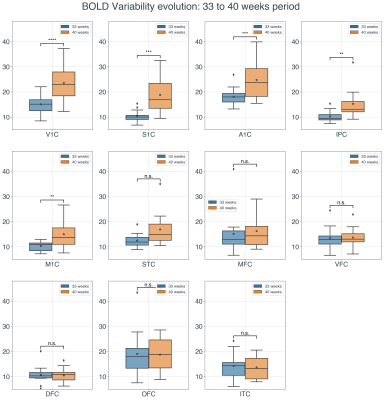
Figure 2: The longitudinal changes of the BOLD variability for the 33- to 40-weeks periods.
The statistical annotation indicates whether the BOLD SD was significantly increased
(after FDR correction) at 40 weeks compared to 33 weeks period (****:
P<0.0001, ***: P<0.001, **: P<0.01). Y-axis represents the BOLD SD
values.
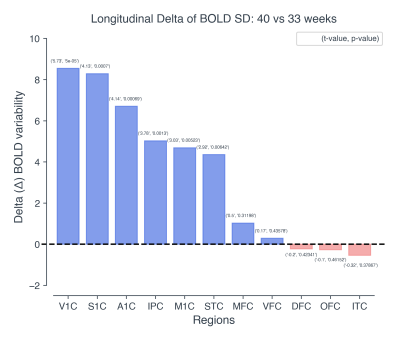
Figure 3: Bar plot showing the longitudinal delta
changes of the BOLD variability for birth (33 weeks) to term-equivalent-age (40
weeks). Values in the parenthesis correspond to the actual t and p values of
the statistical test. The height of each bar in the plot, represents the
estimated delta change (40 vs 33) in the BOLD variability. Blue: positive
change, Red: negative change.
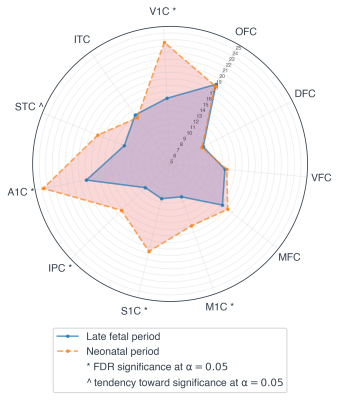
Figure 4: Longitudinal changes of the BOLD variability (SD) between 33- and 40-weeks
time-points. Based on the statistical test, the BOLD
variability in the V1C, M1C, S1C, IPC and A1C regions was significantly
increased at term-equivalent-age compared to 33 weeks period. Furthermore, a
tendency towards statistical significance was observed for the STC region.
DOI: https://doi.org/10.58530/2022/3893