3878
Probing evidence of brain macrostructural disruptions in coronary artery disease: A diffusion MRI tractometry study1Lawson Imaging, Lawson Health Research Institute, London, ON, Canada, 2Medical Biophysics, Western University, London, ON, Canada, 3Cardiology, Western University, London, ON, Canada, 4Lilibeth Caberto Kidney Clinical Research Unit, London Health Sciences Centre, London, ON, Canada, 5School of Kinesiology, Western University, London, ON, Canada
Synopsis
White matter (WM) breakdown is linked to cognitive impairment in coronary artery disease (CAD). We used diffusion MRI tractometry to assess the effects of cardiac disease, brain aging, and cardiac rehabilitation (CR) on regional WM macrostructure in brains of CAD patients. WM disruptions were found in CAD patients at baseline (pre-CR) compared to controls and in old controls compared to young controls. WM improvements, especially in regions linked to cognition, were observed in CAD patients following CR. Both cardiac disease and brain aging may contribute to WM dysfunction in CAD, and these WM disruptions may be favourably modified by CR.
INTRODUCTION
Approximately three in five patients with coronary artery disease (CAD) develop cognitive impairment, the precursor to dementia.1 Breakdown of the brain’s white matter (WM) has been associated with cognitive impairment in CAD, with cognitive decline further accelerating in patients who have had a major cardiovascular event such as a myocardial infarction (MI).2 To facilitate suitable intervention strategies, further investigation of the effect of cardiac disease and brain aging on cerebral WM macrostructure in CAD is needed. Diffusion tensor imaging (DTI) to characterize cerebral WM macrostructure using DTI scalars fractional anisotropy (FA), mean diffusivity (MD), and radial diffusivity (RD) can provide quantitative measurements of WM dysfunction in vascular diseases.3 Specifically, decreases in FA, increases in MD, and increases in RD have been shown to indicate breakdown of cerebral WM pathways in patients with vascular cognitive impairment4 and dementia5. Recent studies have proposed using novel advanced WM fiber quantification techniques, such as tractometry, to probe changes in DTI indices along WM bundles of interest6, which may shed further insight into regional WM dysfunction in vascular disease. However, the feasibility of tractometry in assessing cerebral WM macrostructure related to vascular injury is yet to be investigated. Previously, we used tract-based spatial statistical analysis of DTI indices to assess brain WM macrostructure in CAD patients who had a recent MI and found widespread disruptions in WM regions linked to cognition.3 More importantly, we found that these WM disruptions were favourably modified in CAD patients who participated in a 6-month cardiac rehabilitation (CR) program.3 To further explore WM macrostructural changes in CAD, in this study, we used diffusion MRI tractometry analysis of DTI indices to assess the effects of cardiac disease, brain aging, and CR on regional WM macrostructure in the brains of CAD patients.METHODS
A 10-minute brain diffusion-weighted imaging (DWI) scan was acquired in 35 CAD patients (ten females; mean age = 58 ± 8 years), 21 age-matched healthy control subjects (HCs) (nine females; mean age = 58 ± 9 years), and 25 younger HCs (14 females; mean age = 26 ± 4 years) using a Siemens 3T Verio scanner. 19/35 (54%) CAD patients were re-scanned after participating in a 6-month CR program including low-to-moderate intensity aerobic exercise training. DWI acquisition protocol: single-shot echo-planar imaging with 64 diffusion-encoding directions; b-values = 0 and 1000 s/mm2; 2 mm3 isotropic voxels. Preprocessing and tensor-fitting of each subject’s DWI data were performed as previously described7 to generate FA, MD, and RD maps. Each subject’s DTI scalar maps were spatially normalized to a 1.5-mm Montreal Neurological Institute FA template using the FLIRT tool in FSL8. Diffusion tractography preprocessing steps were then completed using the constrained spherical deconvolution (CSD) algorithm in MRtrix39 as previously described7 to generate CSD peaks images for tractography and tractometry analysis. Tractography and tractometry were performed on the CSD peaks images in TractSeg10 to automatically segment each subject’s brain into 72 WM bundles. Each WM bundle was reconstructed using 5000 streamlines via probabilistic tractography. Tractometry was performed by evaluating DTI indices (FA, MD, RD) at 100 equidistant points along each WM bundle. Group-wise tractometry analysis was then conducted to compare DTI indices in each WM bundle11 between: 1) CAD patients at baseline and age-matched HCs (cardiac disease-related effect); 2) Old HCs and young HCs (age-related effect); and 3) CAD patients pre-CR and post-CR (CR-related effect), while removing confounding effects of sex. p<0.05 was considered significant after family-wise error (FWE) multiple-comparisons correction.RESULTS
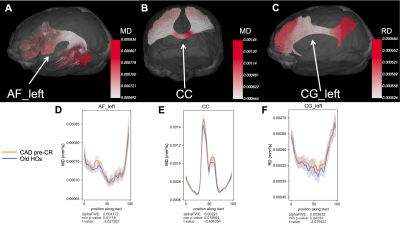
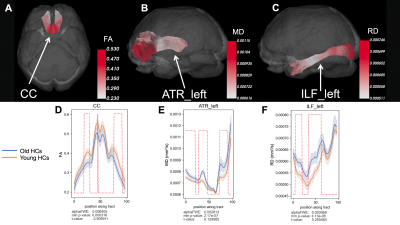
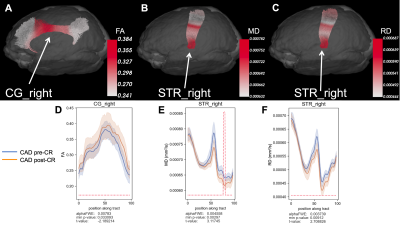
At baseline, CAD patients showed signs of increased MD in the corpus callosum (CC) and left arcuate fasciculus (AF) and increased RD in the left cingulum (CG) compared to age-matched HCs (p < 0.05, uncorrected) (Figure 1). Old HCs had decreased FA in the CC, increased MD in the left anterior thalamic radiations (ATR), and increased RD in the left inferior longitudinal fasciculus (ILF) compared to young HCs (p < 0.05, FWE-corrected) (Figure 2). A potential favourable effect of exercise training was seen in CAD patients post-CR as evidenced by decreased MD in the right superior thalamic radiations (STR) (p < 0.05, FWE-corrected) and increased FA in the right CG and decreased RD in the right STR (p < 0.05, uncorrected) (Figure 3), suggesting potential neuroplastic effect of CR on WM macrostructure.DISCUSSION AND CONCLUSION
In this study, diffusion tractometry revealed subtle changes in WM macrostructure in CAD related to ischemic cardiac injury, brain aging, and potential of CR to favourably modify these adverse brain changes. We found regional changes in WM integrity, especially in WM regions linked to cognition, between CAD patients and controls and between old and young controls, suggesting both cardiac disease and brain aging may contribute to WM impairment in CAD. Interestingly, in CAD patients post-CR, we observed improvements in WM integrity in the CG and STR – important in regulation of autonomic functions and cognition (i.e. memory, attention, and information processing)12. These findings suggest that CR could play an important role in favourably modifying autonomic dysfunction and mitigating damage to brain regions linked to higher order cognitive control in CAD. Next steps to correlate these DTI findings to cognitive performance are underway to better understand WM-related cognitive decline in CAD.Acknowledgements
This work was supported by funding from the Heart and Stroke Foundation of Ontario (JKS and UCA) and from Western University Department of Medicine Program of Experimental Medicine (NS). The authors thank Tim Hartley for his role in coordination of the study and John Butler for assistance with MR imaging.References
1. Burkauskas J, Lang P, Bunevičius A, et al. Cognitive function in patients with coronary artery disease: A literature review. J Int Med Res 2018;46:4019–31.
2. Xie W, Zheng F, Yan L, et al. Cognitive Decline Before and After Incident Coronary Events. Journal of the American College of Cardiology 2019;73:3041–50.
3. Poirier SE, Suskin N, St. Lawrence KS, et al. Cardiac disease may exacerbate age-related white matter disruptions: improvements are feasible after cardiac rehabilitation. Proc Intl Soc Mag Reson Med 2021;29:0385.
4. Chen H-J, Gao Y-Q, Che C-H, et al. Diffusion Tensor Imaging With Tract-Based Spatial Statistics Reveals White Matter Abnormalities in Patients With Vascular Cognitive Impairment. Front Neuroanat 2018;12:53.
5. Huang H, Fan X, Weiner M, et al. Distinctive disruption patterns of white matter tracts in Alzheimer’s disease with full diffusion tensor characterization. Neurobiology of Aging 2012;33:2029–45.
6. Rheault F, Houde J-C, Descoteaux M. Visualization, Interaction and Tractometry: Dealing with Millions of Streamlines from Diffusion MRI Tractography. Frontiers in Neuroinformatics 2017;11:42.
7. Poirier SE, Kwan BYM, Jurkiewicz MT, et al. 18F-FDG PET-guided diffusion tractography reveals white matter abnormalities around the epileptic focus in medically refractory epilepsy: implications for epilepsy surgical evaluation. European Journal of Hybrid Imaging 2020;4:10.
8. Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage 2009;45:S173–86.
9. Tournier JD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 2019;202:116137.
10. Wasserthal J, Neher P, Maier-Hein KH. TractSeg - Fast and accurate white matter tract segmentation. NeuroImage 2018;183:239–53.
11. Yeatman JD, Dougherty RF, Myall NJ, et al. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PLOS ONE 2012;7:e49790.
12. Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: Relations with age and aging. Neuroscience & Biobehavioral Reviews 2015;54:29–37.
Figures