3864
Deep Learning Driven EMI Prediction and Elimination for RF Shielding-Free MRI at 0.055T and 1.5T1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
All clinical MRI scanners require bulky and enclosed RF shielding rooms to prevent external electromagnetic interference (EMI) signals during data acquisition, and quality electronics inside shielding room (i.e., with minimal EMI emission). A deep learning EMI cancellation strategy is presented to model, predict and remove EMI signals from acquired MRI signals, eliminating the need for RF shielding. We demonstrated that this method worked robustly for various EMI sources from both external environments and internal scanner electronics, producing final image SNRs highly comparable to those obtained using a fully enclosed RF shielding cage in 0.055T and 1.5T experiments.
Introduction
Clinical MRI scanners all require bulky and enclosed radiofrequency (RF) shielding cage in practice to prevent external EMI signals during MRI scanning. They also require all scanner electronics within shielding cage to be of high quality (i.e., minimal EMI emission). Several solutions have been recently proposed to tackle the EMI shielding cage requirement for ultra-low-field MRI1-5. An analytical approach was proposed to estimate EMI signal in MRI receive coil from EMI signals detected by EMI sensing coils based on frequency domain transfer functions among coils3. It was further extended for time domain implementation as linear convolutions together with an adaptive procedure4,5, but could produce satisfactory brain imaging results only when used together with conductive cloth and body surface electrode for EMI pickup. Moreover, active EMI removal has never been attempted for 0.1T above. In this study, we present a novel EMI prediction and elimination strategy based on deep learning. We implemented and tested this method for various external and internal EMI sources on (i) an ultra-low-field 0.055T head scanner in absence of any RF shielding and (ii) a 1.5T whole-body scanner with incomplete RF shielding. We validated this method by comparing to the ground truth scenario (i.e., with fully enclosed RF shielding cage in place), and demonstrated its highly effective EMI removal capability.Methods
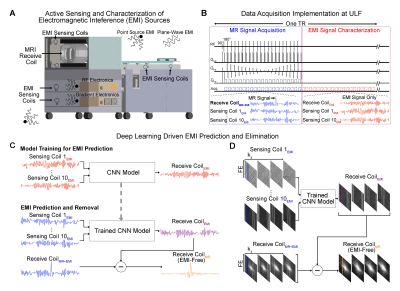
Deep Learning Driven EMI Prediction and EliminationThe deep learning EMI prediction and elimination strategy is illustrated in Figure. 1 for a home-made 0.055T head scanner. Within each TR, MRI receive coil and 10 EMI sensing coils are used to simultaneously sample data within two windows, one is for conventional MRI signal acquisition, the other is for acquiring EMI characterization data (but not MRI signals, i.e., EMI signals only) (Figure. 1B). After each scan, datasets sampled by both MRI receive coil and EMI sensing coils within the EMI characterization window are used to train a five-layer CNN model that can relate the 1D temporal EMI signals received by multiple EMI sensing coils to the 1D temporal signal received by MRI receive coil for each frequency encoding (FE) line (Figure. 1C). This model is then applied to predict EMI signal component in MRI receive coil signal for each FE line within MRI signal acquisition window based on the EMI signals simultaneously detected by EMI sensing coils. Subsequently, the predicted EMI signal component is subtracted from the MRI receive coil signal, creating EMI-free k-space data prior to image reconstruction (Figures. 1C and 1D).
0.055T and 1.5T Experiments
Phantom and Brain datasets were acquired on the shielding-free 0.055T MRI scanner with 1 MRI receiving coil and 10 EMI sensing coils. T2W and FLAIR-like datasets were acquired using 3D FSE with BW=10kHz, and NEX=2.
Phantom datasets were also acquired on a 1.5T whole-body MRI scanner using a 16-channel coil with RF shielding room door open. Four defective channels (receiving little MR signals) were used as EMI sensing coils. 2D GRE T1W datasets were acquired with TR/TE=420/9ms, BW=25kHz, acquisition matrix=200×200×20, and NEX=2.
The complex data sampled within EMI characterization window were split for training (85%) and validation (15%). Each layer within the CNN model was a combination of convolution, batch normalization and rectified linear unit, except the last layer where only convolution operation was performed. The loss function was mean squared error. Typical training time for 0.055T T2W/FLAIR-like and 1.5T T1W datasets were around 5mins and 2mins, respectively.
Results
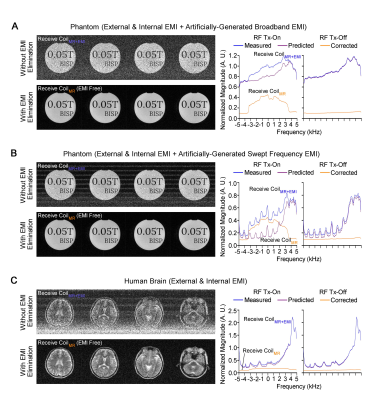
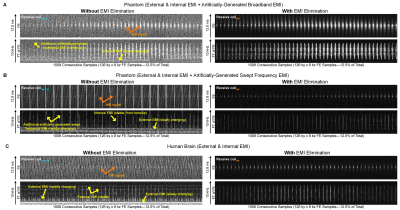
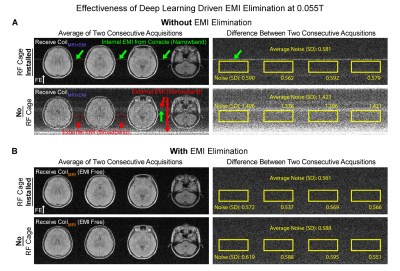
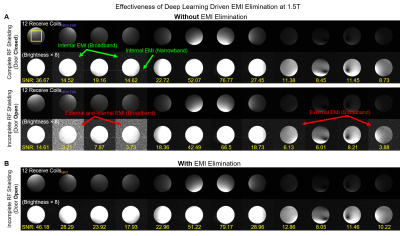
Figures. 2-5 show EMI elimination results of our proposed method. For both phantom imaging and brain imaging (of a cohort of ~100 normal volunteers and patients) at 0.055T with no RF shielding, our method reliably eliminated undesirable EMI signals (Figure. 2), even when EMI sources and their spectral characteristics changed dynamically during scanning (Figure. 3). Figure. 4 demonstrates that the proposed method achieved nearly complete removal of EMI signals in 0.055T brain experiments, producing final image noise levels as low as those obtained using a fully enclosed RF shielding cage (within 5% range).For phantom experiments at 1.5T, Figure. 5 shows the image SNRs6 with and without the active EMI elimination. The results demonstrate that the proposed method produced phantom image SNRs generally better than those with RF shielding room door closed, indicating its ability to remove EMI from both external environments and internal sources.
Discussion and Conclusions
During scanning, EMI signals can change dynamically due to surrounding EMI sources of various nature and behaviours (e.g., nearby EMI source relocations, as well as patient body position changes because EMI signal received by MRI receive coil can be influenced by the position/shape of human body that serves as an effective antenna for EMI pickup7,8). Here we developed a deep learning driven EMI prediction and elimination strategy for MRI with no or incomplete RF shielding. The nature of deep learning was expected to enable a versatile model that robustly establishes the relationships among external and internal EMI signals detected by EMI sensing coils and MRI receive coil, especially for point-of-care MRI operations. This hypothesis has been well supported by the robust 0.055T results from a large cohort of normal volunteers/patients. Furthermore, our 1.5T results clearly indicated the general applicability of our method beyond ultra-low-field.Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (R7003-19F, HKU17112120 and HKU17127121 to E.X.W., and HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L.), and Lam Woo Foundation to E.X.W..References
[1] Rearick T, Charvat GL, Rosen MS, Rothberg JM; Noise suppression methods and apparatus. (US Patent No. 9,797,971). U.S. Patent and Trademark Office. 2017.
[2] Dyvorne H, Rearick T, Poole M, Lazarus C, Weiss P, Sacolick L, Jordan J, Hugon C, Mileski W, Chen G, O'Halloran R, McNulty C, Lowthert J, Nelson A, Lorenzo A, Zwart N, Kundu P, Martin S, Loutchouk A, Welch EB, By S, Cahn B, Yuen M, Mazurek M, Pranhat A, Rosen M, Sheth K, Rothberg J. Freeing MRI from its Faraday cage with Interference Rejection. In: Proceedings of the 29th Annual Meeting of ISMRM, 2021, p 0749.
[3] Srivinas SA, Cooley CZ, Stockmann JP, McDaniel PC, Wald LL. Retrospective Electromagnetic interference mitigation in a portable low field MRI system. In: Proceedings of the 28th Annual Meeting of ISMRM, 2020, p 1269.
[4] Srivinas SA, Cauley S, Stockmann JP, Sappo CR, Vaughn CE, Wald LL, Grissom WA, Cooley CZ. In vivo human imaging on a 47.5mT open MRI system with active Electromagnetic Interference (EMI) mitigation using an electrode. In: Proceedings of the 29th Annual Meeting of ISMRM, 2021, p 4030.
[5] Srinivas SA, Cauley SF, Stockmann JP, Sappo CR, Vaughn CE, Wald LL, Grissom WA, Cooley CZ. External Dynamic InTerference Estimation and Removal (EDITER) for low field MRI. Magn Reson Med 2021.
[6] Xie VB, Lyu M, Wu EX. EPI Nyquist ghost and geometric distortion correction by two‐frame phase labeling. Magn Reson Med 2017;77(5):1749-1761.
[7] Kibret B, Teshome AK, Lai DT. Analysis of the human body as an antenna for wireless implant communication. IEEE Transactions on antennas and propagation 2016;64(4):1466-1476.
[8] Sen S, Maity S, Das D. The body is the network: to safeguard sensitive data, turn flesh and tissue into a secure wireless channel. IEEE Spectrum 2020;57(12):44-49.
Figures