3856
A Nitroreductase Responsive Fluorescent/ 19F MRI Dual-Imaging Probe for Hypoxia Tumor Detection In Vivo1Key Laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences–Wuhan National Laboratory for Optoelectronics, Wuhan, China
Synopsis
We designed and synthesized a molecular probe-based on fluorescence imaging and 19F MRI bimodal imaging to identify overexpressed nitroreductase in hypoxic tumor. The two imaging modes complement each other in sensitivity and imaging depth, providing more abundant information. More importantly, the bimodal molecular probe is enriched in the tumor region depending on the passive targeting ability and has been well verified by fluorescence imaging and 19F MRI in the metastatic tumor model. And we successfully identified the cancerous left lung and healthy right lung in lung cancer model mouse.
Introduction
Hypoxia, a condition of inadequate oxygen supply at the tissue level, is one typical characteristic of several solid tumors [1]. Generally, NTR as a biomarker detection represents a very important detection method for hypoxia as the high selectivity and sensitivity. Although great progress has been made in the fluorescent detection of NTR, inherent poor tissue penetration depth and low targeting limit the scope of applications [2]. The synergistic combination of two or more imaging modalities provides solutions able to address multiple issues such as sensitivity and tissue penetration efficiency [3].Methods
To developed a dual-modality NTR-responsive probe, herein, we choose Cy7 skeleton as the fluorescence reporting unit. A -NO2 group fused to indole of the Cy7 skeleton was chosen as the NTR recognition unit as inspired by previous NTR-selective fluorescent probe. To add the ability that responsive based on MRI, a –F group was added onto two symmetric indole groups. To increase the solubility of Cy7, sulfonated form Cy7 was synthesized.Results and Discussion
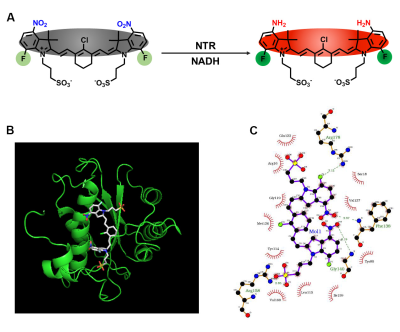
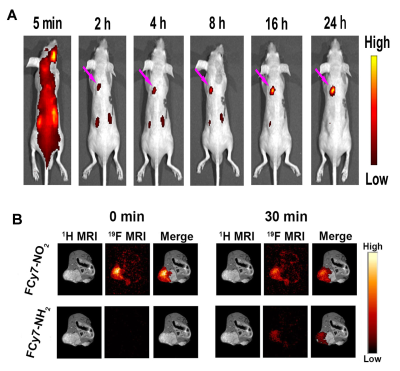
Nitroreductase, a cytosolic enzyme, can make use of NADH as an electron source to reduce the nitro groups to the corresponding amines (Figure 1A). To get the deep understanding into the relationship between structure and NTR detection ability, docking calculations were carried out for FCy7-NO2 with nitroreductase. The aromatic nitro part of FCy7-NO2 is more inclined to close to the hydrophobic region of NTR (Figure 1B). A large docking value of probe FCy7-NO2 indicated its high bindingaffinity to NTR while a low value of intermediates mean that substrates could dissociate from enzyme easily (Figure 1C). After injecting FCy7-NO2 via retro-orbital, the signal intensity of lung has been increased in suit lung cancer mouse (Figure 2A). We next sought to apply FCy-7-NO2 to molecular imaging of NTR release in metastatic tumor model mice. With the diffusion of the contrast agent in the tumor area and the prolongation of the enzymatic action, the FCy7-NO2 signal decreased by about 33% and the signal of the target product gradually appeared (Figure 2B).Conclusion
Compared with conventional single optical detection, we proposed using NIR fluorescence imaging and 19F NMR/MRI to identify NTR in vitro and in vivo. Successively, the FCy7-NO2 probe is applied as bimodal contrast agent for the combined NIR fluorescence imaging and 19F MRI of A549 cells and tumor-bearing mice. Specifically, using 19F detection method, the concentration of the NTR can be quantified in higher concentration range which is far beyond the fluorescent detection limit. This work also demonstrated that incorporation of multiple stimuli-responsiveness into multimodal probes would inspire new interests for the design of smart probes for other biochemical processes, including other enzymatic activities, small molecules and ions.Acknowledgements
This work is supported by National Key R&D Program of China (2018YFA0704000), and National Natural Science Foundation of China (81625011, 91859206, 81730048, 81971705).References
[1] Brown J, Wilson W, et al. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437-447.
[2] Li Y, Sun Y, Li J, et al. Ultrasensitive Near-Infrared Fluorescence-Enhanced Probe for in Vivo Nitroreductase Imaging. J Am Chem Soc. 2015;137(19):6407-6416.
[3] Li Y, Zhang H, Guo C, et al. Multiresponsive Nanoprobes for Turn-On Fluorescence/ 19F MRI Dual-Modal Imaging. Anal Chem. 2020;92(17):11739-11746.
Figures