3854
Simultaneous macromolecular proton fraction and proton density fat fraction quantification using chemical shift-encoding based spin-lock MRI1Department of Imaging and Interventional Radiology, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 2Illuminatio Medical Technology Limited, Hong Kong, Hong Kong, 3Philips Healthcare, Hong Kong, Hong Kong, 4Philips Healthcare, Shenzhen, China, 5Department of Medicine & Therapeutics, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 6Department of Diagnostic and Interventional Radiology, Technical University of Munich, Munich, Germany
Synopsis
Chronic liver disease is a major healthcare problem worldwide. Liver fibrosis and fat fraction are two main features of chronic liver diseases. Recent work reported that macromolecular proton fraction (MPF) mapping has potential for diagnosis of liver fibrosis. In this work, we reported a novel technique to quantify MPF and fat fraction of the liver simultaneously in a brief breath hold.
Introduction
Chronic liver disease is a major healthcare problem worldwide. Steatosis and fibrosis are main diagnostic and prognostic features of chronic liver diseases. Macromolecular proton fraction (MPF) represents the relative amount of protons associated with macromolecules involved in magnetization exchange with free water protons. It has been reported that MPF has the potential to detect liver fibrosis1-3. In our previous work, we proposed to measure MPF using spin-lock MRI2. In this approach, Spectral Presaturation with Inversion Recovery (SPIR) was used for fat suppression. For fatty liver disease with excessive fat content, coupled with significant B1 RF and B0 field inhomogeneities, we expect the residual fat signal after SPIR can lead to quantification errors of MPF. In this work, we propose a chemical‐shift encoding–based water–fat separation method with multifrequency fat spectrum modeling using spin‐lock MRI. The purpose of this work has two folds: (1) to improve accuracy of MPF quantification in presence of excessive fat content; (2) to rapidly obtain co-registered MPF and PDFF in the liver in a single breath-hold.Methods
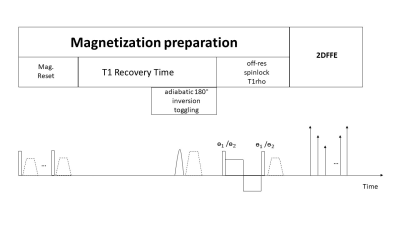
Figure 1 shows the pulse sequence diagram. The sequence is designed based on spin-lock prepared fast/turbo field echo (gradient echo) acquisitions with MPF signal encoded using the spin-lock preparation, as described in our previous work2. A 6-peak fat model is used for water-fat separation and PDFF quantification. We previously demonstrated the coefficients of each fat peak is highly depended on the spin-lock RF pulse cluster due to its sensitivity to chemical shift and B0 field inhomogeneity4. In this work, we used a pre-calibration approach to obtain all fat coefficients for our off-resonance spin-lock RF pulse cluster. These coefficients are then used with Iterative Decomposition of water and fat with Echo asymmetry and Least-squares estimation (IDEAL)5 for water-fat separation and fat quantification. MPF map is calculated from water images after IDEAL reconstruction.Simulation study was conducted to compare the accuracy of MPF quantification by using SPIR-based fat suppression and the proposed approach in the presence of excessive fat content. The numerical phantom used in the simulation includes a region of pure fat, a region of pure water, and a region with a mix of fat and water with fat fraction 50% (Figure 2). Simulation parameters include: frequency of spin-lock (FSL) 400 Hz, frequency offset (FO) 4000 Hz for the first spin-lock group; FSL 100 Hz and FO 1000 Hz for the second spin-lock group; time of spin-lock (TSL) 50ms; TR/TE1/ ΔTE/nTE 6.3 ms/1.04 ms/0.8 ms/6.
In vivo study was conducted under the approval of the institutional review board. All scans were conducted using a 3.0 T MRI scanner (Achieva TX, Philips Healthcare). A 32-channel cardiac coil was used as the receiver and the body coil was used as the RF transmitter. Sequence parameters are same as those used in the simulation. Acquisition resolution was 2.5×2.5×7 mm3. The imaging data of the proposed method was acquired within a single breath-hold of 14 seconds. We also used the MPF mapping method described in previous work2 to obtain MPF, and the vendor provided mDixon QuantTM (Philips Healthcare) to obtain PDFF. The separately acquired MPF and PDFF were used as references and compared to those from the proposed method.
Results
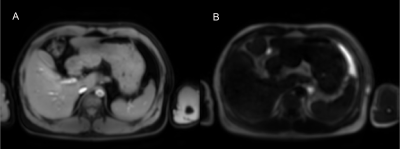
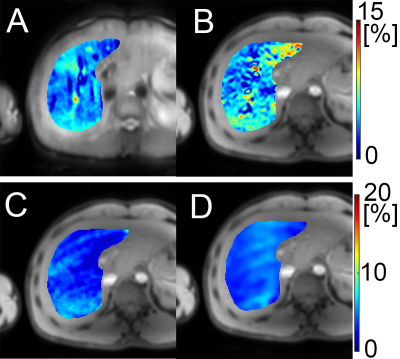
Figure 2 show the simulation results. Note the proposed method had significantly reduced MPF quantification errors compared to the SPIR-based fat suppression method at the regions with mixed water and fat. Figure 3 show the water image and fat image from the in vivo scan after reconstruction using the proposed method. Figure 4 show the MPF map and the PDFF map from the proposed method, and the reference MPF and PDFF map. Note the PDFF results from the proposed method was consistent with the reference results. The MPF measured using the proposed method was consistent with the reference in the right lobe of the liver.Discussion
In our previous work, we have demonstrated chemical shift-encoding method for simultaneous water-fat separation and T1rho quantification using on-resonance spin-lock4. The hard RF pulse based spin-lock is highly sensitive to chemical shift effects when performed on-resonance, which leads to challenges to obtain coefficient of each fat peak for water-fat imaging. Adiabatic RF pulse approach was previously proposed to address this problem4.In this work, to encode MPF signal, the spin-lock is performed at far off-resonance. We used the hard RF pulse based spin-lock in this work since it is insensitive to chemical shift effects. It is worthy of further investigation whether adiabatic spin-lock has advantages in off-resonance spin-lock. The proposed method is based on TFE/FFE (gradient echo) acquisition. The previously reported MPF mapping is based on fast spin echo acquisition. Further study is needed to compare FSE and TFE/FFE in quantitative imaging in the liver.
Conclusion
We reported a technique to for simultaneous quantification of MPF and fat content in the liver. The imaging data sets can be acquired within a single breath-hold of 14 seconds. The method has potential to improve MPF quantitation in patients with severe fatty liver diseases. The fully registered PDFF map and MPF map can be used for multi-parametric analysis of chronic liver diseases.Acknowledgements
This study was supported by a grant from the Innovation and Technology Commission of the Hong Kong SAR (Project MRP/046/20X), a Faculty Innovation Award from the Chinese University of Hong Kong, and a grant from the Research Grants Council of the Hong Kong SAR (Project SEG CUHK02).References
1. Yarnykh, V.L., Tartaglione, E.V. & Ioannou, G.N. Fast macromolecular proton fraction mapping of the human liver in vivo for quantitative assessment of hepatic fibrosis. NMR in Biomedicine 28, 1716-1725 (2015).
2. Hou J, Wong VW-S, Jiang B, et al. Macromolecular proton fraction mapping based on spin-lock magnetic resonance imaging. Magn Reson Med. 2020;00:1–15
3. Hou, J, Wong VW-S, Wong GL-H, et al. Macromolecular proton fraction mapping based on spin-lock for the non-invasive diagnosis of early stage fibrosis. Proceedings of the 30th Annual Meeting of ISMRM. 0314 (2021).
4. Chen, W. & Karampinos, D.C. Chemical-shift encoding–based water–fat separation with multifrequency fat spectrum modeling in spin-lock MRI. Magnetic Resonance in Medicine 83, 1608-1624 (2020).
5. Reeder SB, McKenzie CA, Pineda AR, et al. Water–fat separation with IDEAL gradient‐echo imaging. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2007 Mar;25(3):644-52.
Figures