3851
Amide Proton Transfer Imaging of Bladder Cancer at 3 T: A Preliminary Study1Tongji Hospital, School of Medicine, Tongji University, Shanghai, China, 2Philips Healthcare, Shanghai, China
Synopsis
Amide proton transfer (APT) imaging is an emerging chemical exchange saturation transfer (CEST)-based MRI technique that is sensitive to mobile proteins and peptides in tissue and has drawn considerable attention in the field of cellular and molecular imaging. In the present work, our result demonstrates that it is feasible to use APT imaging for Bladder Cancer (BCa) and it has shown great potential for bladder cancer imaging, thus opening new research avenues in this field.
Introduction
In the past years, studies have demonstrated essential roles of magnetic resonance imaging in bladder cancer diagnosis, grade and stage. However, these morphological and functional multiparameter MRI sequences cannot reflect the pathophysiology of the tissue at the molecular level, and given the limitations of current clinical approaches, new molecular MRI techniques are needed for Bladder cancer. Amide proton transfer (APT) imaging is an emerging chemical exchange saturation transfer (CEST)-based MRI technique1 that is sensitive to mobile proteins and peptides in tissue and has drawn considerable attention in the field of cellular and molecular imaging. APT imaging provides exciting insights into the pathogenesis of cancer, as tumors present an increased number of cellular proteins and peptides compared with normal, healthy tissue. Many studies have investigated brain tumors2-5, stroke3, prostate cancer6, endometrial cancer7, cervical cancer8,9, rectal cancer10,11, breast cancer12 and thoracic tumors13 by APT imaging and found that it has potential clinical application value for detecting tumors, differentiating between benign and malignant lesions, providing tumor grading, and evaluating the efficacy of chemotherapy. However, there have not yet been any reports testing the feasibility of APT in BCa; therefore, we aimed to explore whether APT imaging could be applied for BCa. In this study, we established the reliability of APT imaging in healthy volunteers and patients with BCa.Method
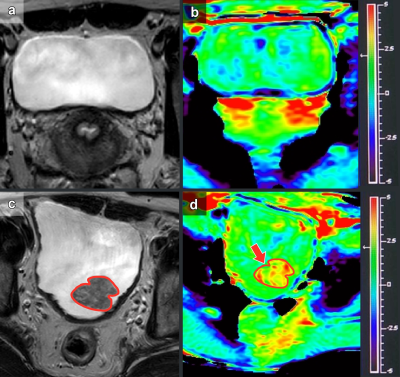
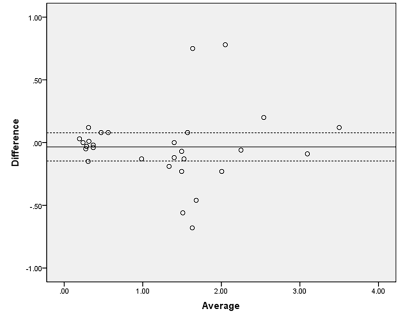
Eleven healthy volunteers and 18 patients pathologically confirmed to have BCa were enrolled in this study between March 2021 and September 2021. One patient with small lesion (diameter less than 5 mm) and patients (n=2) with pathologically confirmed benign lesions were excluded. All patients were subjected to pelvic MRI at 3.0 T (Ingenia; Philips Healthcare) with a Torso coil. The asymmetric magnetization transfer ratio (MTRasym) was assessed in 11 healthy volunteers and 18 patients with bladder cancer. The intraclass correlation coefficient (ICC) was used to evaluate the interobserver agreement of all metrics. APT parameter repeatability was assessed by paired-samples t tests and Bland-Altman tests. Nonparametric tests compared the differences between healthy volunteers and patients with bladder cancer.Results
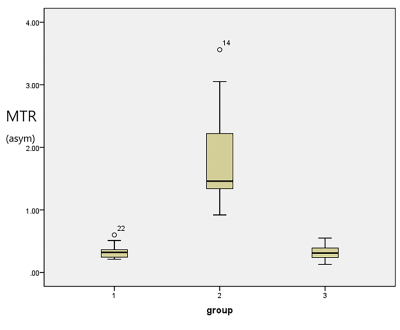
MTRasym showed good consistency and repeatability for all subjects. The MTRasym in patients with bladder cancer was significantly higher (1.46 [0.95]) than that in the bladder walls of healthy volunteers (0.34±0.12) and patients with bladder cancer (0.31±0.11) (P<0.05). There was no significant difference in the bladder walls between healthy volunteers and patients with bladder cancer.Discussion
The reliability of APT imaging in healthy volunteers and patients with BCa was established. These results showed that the APT parameters of healthy volunteers and patients with BCa have good consistency and repeatability. The presented assessment of APT imaging variability of patients with BCa and healthy volunteers at 3.0 T suggested the feasibility of further exploration of this novel technique to examine BCa pathology. The MTRasym values of patients with BCa were higher than those from the bladder walls of healthy volunteers and the normal bladder walls of patients with BCa. There was no significant difference between the bladder walls of healthy volunteers and the normal bladder walls of patients with BCa. BCa shows obvious atypical cells and structures. These observations may confirm that BCa cells proliferate more actively, and the cytoplasmic protein and peptide contents increase significantly compared with normal urothelial tissue. Moreover, it has been reported that APT imaging could be used to grade brain tumors2-5, prostate cancer6, endometrial cancer7, cervical cancer8,9, and rectal cancer10,11. Thus, it could be feasible to use APT imaging for BCa grading and staging. APT imaging has shown great potential value in BCa, and its ability to evaluate the grade and invasiveness of BCa will be investigated in future studies.Conclusion
In conclusion, it is feasible to use APT imaging for BCa and it has shown great potential for BCa imaging.Acknowledgements
This work was funded by National Natural Science Foundation of China, contract grant numbers: Program No. 81901733, 81974274; Excellent Discipline Reserve Talent Program of Tongji Hospital Affiliated to Tongji University , Grant No. HBRC2109; Key discipline construction project of the three-year action plan of Shanghai public health system, No. GWV-10.1-XK9; Shanghai "Rising Stars of Medical Talent" Youth Development Program-Youth Medical Talents-Medical Imaging Practitioner Program, Grant No. SHWRS(2020)_087.
References
1. Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 2003;9:1085-1090.
2. Zhou J, Tryggestad E, Wen Z, et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and Peptides. Nature Med 2011;17:130-134.
3. Zhao X, Wen Z, Huang F, et al. Saturation power dependence of amide proton transfer image contrasts in human brain tumors and strokes at 3T. Magn Reson Med 2011;66: 1033-1041.
4. Yuan J, Chen S, King AD, et al. Amide proton transfer-weighted imaging of the head and neck at 3 T:a feasibility study on healthy human Subjects and patients with head and neck cancer. NMR Biomed 2014;27: 1239-1247.
5. Zhou J, Zhu H, Lim M, et al. Three-dimensional amide proton transfer MR imaging of gliomas: initial experience and comparison with gadolinium enhancement.J Magn Reson Imaging 2013;38: 1119-1128.
6. Jia G, Abaza R, Williams JD, et al. Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson Imaging 2011;33:647-654.
7. Takayama Y, Nishie A, Togao O, et al. Amide Proton Transfer MR Imaging of Endometrioid Endometrial Adenocarcinoma:Association with Histologic Grade. Radiology 2018;286:909-917.
8. Li B, Sun H, Zhang S, Wang X, Guo Q. Amide proton transfer imaging to evaluate the grading of squamous cell carcinoma of the cervix: A comparative study using 18 F FDG PET. J Magn Reson Imaging 2019;50:261-268.
9. Li B, Sun H, Zhang S, Wang X, Guo Q. The utility of APT and IVIM in the diagnosis and differentiation of squamous cell carcinoma of the cervix: A pilot study. Magn Reson Imaging 2019; 63:105-113.
10. Nishie A, Takayama Y, Asayama Y, et al. Amide proton transfer imaging can predict tumor grade in rectal cancer. Magn Reson Imaging 2018;51:96-103.
11. Nishie A, Asayama Y, Ishigami K, et al. Amide proton transfer imaging to predict tumor response to neoadjuvant chemotherapy in locally advanced rectal cancer. J Gastroenterol Hepatol 2019;34:140-146.
12. Dula, AN, Arlinghaus, LR, Dortch RD, et al. Amide proton transfer imaging of the breast at 3 T: establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med 2013;70:216-224.
13. Ohno Y, Yui M, Koyama H, et al. Chemical Exchange Saturation: Preliminary Results for Differentiation of Malignant and Benign Thoracic Lesions. Radiology 2016;279:578-589.
Figures