3843
Free-breathing abdominal CEST sequence using water presaturation and precise respiratory synchronization
Zhensen Chen1,2, Chuyu Liu3, Yishi Wang4, Weibo Chen5, Rui Guo6, He Wang1,7, and Xiaolei Song3
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2MOE Frontiers Center for Brain Science, Fudan University, Shanghai, China, 3Department of Biomedical Engineering, School of Medicine Tsinghua University, Beijing, China, 4Philips Healthcare, Beijing, China, 5Philips Healthcare, Shanghai, China, 6Department of Medicine (Cardiovascular Division), Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States, 7Human Phenome Institute, Fudan University, Shanghai, China
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2MOE Frontiers Center for Brain Science, Fudan University, Shanghai, China, 3Department of Biomedical Engineering, School of Medicine Tsinghua University, Beijing, China, 4Philips Healthcare, Beijing, China, 5Philips Healthcare, Shanghai, China, 6Department of Medicine (Cardiovascular Division), Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States, 7Human Phenome Institute, Fudan University, Shanghai, China
Synopsis
To date, chemical exchange saturation transfer (CEST) is rarely used in abdominal imaging of human body due to its high sensitivity to motion. In this study, we developed a free-breathing abdominal CEST sequence that uses a precise respiratory synchronization strategy and includes a water presaturation module. Preliminary in-vivo experiments showed that the proposed sequence outperformed non-triggered and breath-holding abdominal CEST in reducing the slice position inconsistency between the frequency offsets and motion-induced noise.
Introduction
Chemical exchange saturation transfer (CEST) MRI is a promising molecular imaging technique, and has been demonstrated useful in diagnosis of various diseases. To date, CEST is rarely used in abdominal imaging of human body due to its high sensitivity to motion. A few previous studies have used breath-holding to perform abdominal CEST imaging, in which one breath-hold was required for the acquisition of each frequency offset [1-3]. However, there may remain large inconsistency in slice position over the frequency offsets due to the difficulty in achieving the same breathing level, and it would cause uncomfortableness to patients and long total acquisition time when acquiring many frequency offsets. Therefore, this study aimed to develop a free-breathing sequence for abdominal CEST imaging.Methods
Sequence design: The diagram of the proposed sequence is shown in Figure 1. Compared to the conventional CEST sequence, the proposed sequence is unique in two aspects: (1) Precise respiratory synchronization is implemented to ensure that the readout is located inside a pre-designated window of expiratory phase [αlb, αub], where the expiratory phase α is defined as the time interval between readout and start of expiration phase (tacq) divided by the total duration of expiration phase (texp). To achieve this, respiratory triggering is used and the trigger delay (td_tri) is adaptively adjusted according to the predicted total durations of inspiration phase and expiration phase from previous respiratory cycles, such that the readout is placed at a targeted expiratory phase αtar. In addition, immediately after the trigger signal of the subsequent respiratory cycle, the real expiratory phase αreal of the readout is calculated, and data will be re-acquired if αreal falls outside [αlb, αub]. (2) To remove the influence of repetition time variation among respiratory cycles, a water presaturation module that is followed by a delay time (td_wsat) is performed before the CEST saturation module.Simulation: To investigate influence of the water presaturation module on CEST contrast, simulation of the proposed sequence under different delay times after the water presaturation module (i.e. td_wsat) was performed, by using the Bloch-McConnell equation-based simulation code downloaded from http://www.cestsources.org[4].
In-vivo experiments: For proof of concept, preliminary in-vivo experiments of the proposed sequence were performed on two healthy volunteers (males, 23 years and 54 years). Both volunteers provided written informed consent before participation. The experiments were performed on a Philips Ingenia CX 3T MR scanner (Philips Healthcare, Best, The Netherlands), using a 16-channel body coil and a 16-channel posterior coil as the receivers. For all CEST scans, a CEST saturation duration (tsat) of 1800 ms and a single-slice single-shot TSE readout (~300 ms, in coronal direction) were used. For the proposed respiratory synchronization strategy, the targeted expiratory phase αtar and the window [αlb, αub] were set to 0.5 and [0.3, 0.7], respectively.
Results and Discussion
Figure 2 shows the simulated Z-spectra with water presaturation, as well as the conventional one without water presaturation. As can be seen, the Z-spectra with water presaturation are higher than the conventional one at all frequency offsets, and the CEST peak in MTR asymmetry is slightly diminished. This influence of water presaturation probably can be corrected by using the recently proposed quasi-steady CEST analysis approach [5], which will be investigated in the near future.A demonstration of the proposed respiratory synchronization strategy for free-breathing abdominal CEST imaging on volunteer #1 is presented with animation in Figure 3. Compared to the non-triggered one, respiratory triggering did largely reduce the motion-induced slice location inconsistency between the frames, and the proposed precise respiratory synchronization strategy (i.e. the one with strict data check) seems to outperform the conventional one (i.e. the one with simple data check).
A comparison of CEST scans using breath-holding and the proposed respiratory synchronization strategy (i.e. triggered, with strict data check) on volunteer #1 is presented with animation in Figure 4. The proposed strategy is apparently much better than breath-holding in reducing the total acquisition time as well as slice location inconsistency, although there remains one frame with large position shift.
Figure 5 shows the comparison of B0 map, Z-spectrum and MTR asymmetry map between the hepatic CEST scans with non-triggered and the proposed respiratory synchronization strategy on volunteer #2. The proposed method resulted in a more homogeneous MTR asymmetry map with less artifacts on the boundary (white arrows) and fewer noisy oscillations (red arrows) on Z-spectrum.
Conclusion
The proposed free-breathing abdominal CEST sequence with water presaturation and precise respiratory synchronization is feasible and can result in shorter acquisition time and less motion artifact than breath-holding CEST. More solid validations and comparisons for the proposed sequence are on-going.Acknowledgements
This work was supported by the Research Start-up Funds from Fudan University to Dr. Zhensen Chen, National Natural Science Foundation of China (82071914), and the startup package from Tsinghua University to Dr. Xiaolei Song.References
- Chen, S.Z., et al., Chemical exchange saturation transfer (CEST) MR technique for in-vivo liver imaging at 3.0 tesla. Eur Radiol, 2016. 26(6): p. 1792-800.
- Deng, M., et al., Chemical Exchange Saturation Transfer (CEST) MR Technique for Liver Imaging at 3.0 Tesla: an Evaluation of Different Offset Number and an After-Meal and Over-Night-Fast Comparison. Mol Imaging Biol, 2016. 18(2): p. 274-82.
- Tang, Y., et al., Noninvasive Detection of Extracellular pH in Human Benign and Malignant Liver Tumors Using CEST MRI. Front Oncol, 2020. 10: p. 578985.
- Zaiss, M. CEST sources. 2014 [cited 2021 March 1]; Available from: http://www.cest-sources.org.
- Sun, P.Z., Quasi-steady state chemical exchange saturation transfer (QUASS CEST) analysis-correction of the finite relaxation delay and saturation time for robust CEST measurement. Magn Reson Med, 2021. 85(6): p. 3281-3289.
Figures
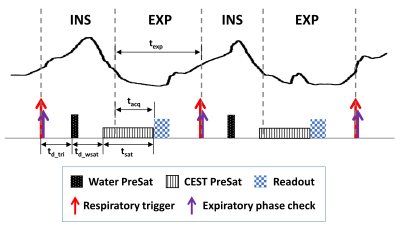
Figure
1. Diagram of the proposed free-breathing abdominal CEST sequence.
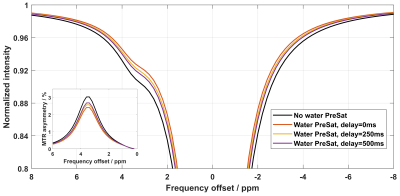
Figure 2. Simulated
z-spectra without water presaturation and with water presaturation using
different delay times. The MTR asymmetry is shown in the inset. Two pools, i.e.
water and amide, were included in the simulation, with the T1 and T2 of tissue set
to 1000 ms and 50 ms, respectively. The B1 power and duration of the CEST saturation
were 0.5 ut and 1800 ms, respectively.
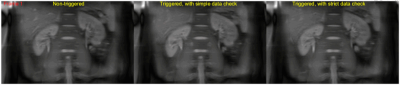
Figure 3.
Comparison of different respiratory synchronization strategies for
free-breathing abdominal CEST imaging on volunteer #1. The strategy “triggered,
with strict data check” corresponds to the one presented in Figure 1, while the
strategy “triggered, with simple data check” means data were accepted as long
as the readout was within the expiration phase. For convenience of comparison,
all frames were acquired under the non-CEST-saturated condition (i.e. using a
frequency offset of -1560 ppm).
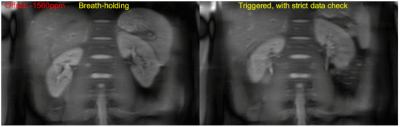
Figure 4. Comparison
of abdominal CEST scans using breathing-holding and the proposed respiratory
synchronization strategy (i.e. triggered, with strict data check) on
volunteer #1. The breath-holding scan took ~16
mins, while the free-breathing scan took ~4min. The key imaging parameters
were: CEST B1 power 1.8 ut, 29 frequency offsets ranging from -5 ppm to 5 ppm,
non-CEST-statured image acquired 4 times, voxel size 2.5×3×6 mm3.
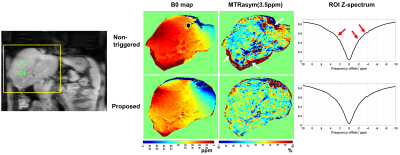
Figure 5. Comparison of B0 map, Z-spectrum and MTR asymmetry map at 3.5 ppm between the
CEST scans using non-triggered and the proposed respiratory synchronization on
volunteer #2. Motion-induced artifacts on MTR asymmetry map and noisy
variations on Z-spectrum are indicated with white and red arrows, respectively.
The B0 maps were obtained by performing voxel-wise single-pool Lorentzian
fitting to the Z-spectra. The key imaging parameters were: delay after water
presaturation 250 ms, CEST B1 power 1ut, 45 frequency offsets ranging from -20
ppm to 20 ppm, voxel size 1.7×1.7×4 mm3.
DOI: https://doi.org/10.58530/2022/3843