3835
Preliminary demonstration of a new motion-based noise reduction method for 3D CEST-MRI
Botao Zhao1 and Xiao-Yong Zhang1
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
Synopsis
Quantification of chemical exchange saturation transfer (CEST) imaging is a keystone for many clinical applications. Head motion and motion-caused noise produce non-negligible effects on the quantification in the whole brain 3D imaging. To solve the problem, we propose a new motion-based noise reduction method. Our preliminary results demonstrate that our method can reduce the noise efficiently and improve the amide proton transfer (APT) quantification results in the conditions of either with slight head motion or with huge head motion.
Introduction
Quantification of chemical exchange saturation transfer (CEST) imaging plays an important role in many clinical and preclinical studies [1]. Motion and motion-caused noise are inevitable during the CEST acquisition process. Recently, several studies have been done on motion correction [2, 3] by moving the images to the correct position. But the motion-caused noise was always overlooked during the CEST quantification. As for the existing noise reduction methods for CEST images, a method of low-rank approximation of structured signals is commonly used, of which the main features can be calculated by the singular value decomposition or the principal component analysis [4]. In principle, this method reduces the Gaussian noise by exploiting the data redundancy, but it is not proper for continuous head motion-caused noise. To address this problem, we propose a new noise reduction method that could reduce both the Gaussian noise and motion-caused noise.Principle and Methods
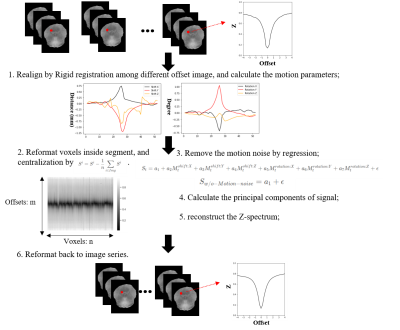
Principle: Our motivation derives from the preprocess of fMRI and we assume that the head motion is linear dependent on the collected signal. Therefore, we apply linear regression to regress out the motion using parameters in six directions. To avoid removing the normal characteristics of Z-spectrum, we do the zero-centred normalization for raw data before regression.Method: As shown in Fig. 1, our method contains six steps. Firstly, we register the images at different offsets using the rigid registration method and get the six motion parameters. Next, we reformat voxels inside the segment and make the data be zero-centered by subtracting the mean value. Then, we reduce the motion noise by linear regression, which is the key step in our method. We suppose that the zero-centered signal is $$S_{t} = a_{1} + a_{2} M^{shift:X}_{t} + a_{3} M^{shift:Y}_{t} + a_{4} M^{shift:Z}_{t} + a_{5} M^{rotation:X}_{t} + a_{6} M^{rotation:Y}_{t} + a_{7} M^{rotation:Z}_{t} + \epsilon,$$ while S_{t} means the signal at offset t. The motion-relation information is considered as the noise. Then, the signal without motion noise is calculated as: $$S_{w/o-motion-noise} = a_{1} + \epsilon.$$ The fourth step is to calculate the principal components of the signal without motion noise and remove the redundant components. The redundant components are considered mainly caused by Gaussian noise. Then, we reconstruct the Z-spectrum by $$Z = \hat{Z} + \sum^{k}_{i} S \varphi_{i} \varphi^{T}_{i}, $$ while the $$$\varphi_{i}$$$ denotes the principal component. Finally, we get the denoised Z-spectrum by reshaping the matrix.
MRI: To evaluate our method, CEST experiments were performed on a 3T whole-body MR scanner (MAGNETOM Prisma, Siemens Healthineers, Erlangen, Germany) using a 64-channel receive head/neck coil and the integrated transmit body coil. Eight health volunteers were included and two subjects were found non-negligible head motion during scanning. After a Localizer scanning for positioning, the whole brain CEST images were obtained by a continuous wave (CW) RF irradiation (B1=0.8 mT and saturation time Tsat=4s) followed by a snapshot sequence to readout with parameters: matrix size =120×144×20, FOV=183×220×100 mm, number of slices = 20. Z-spectra, presented as measured signals ($$$S_{sat}$$$) normalized by a reference signal ($$$S_{0}$$$), were acquired with RF offsets from -50 to 50 Hz (-50, -25, -10, -9 -8, -7, -6, -5, -4.5, -4.25, -4, …, 4, 4.25, 4.5, 5, 6, 7, 8, 9, 10, 25, 50). S0 was obtained by setting the RF offset to -200 Hz.
Results and discussion
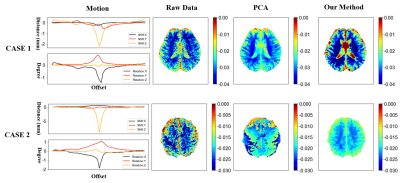
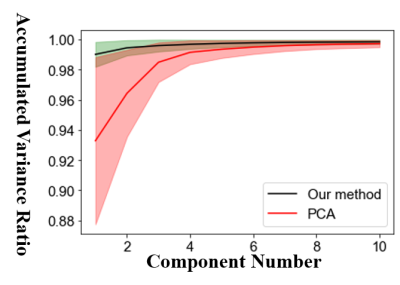
As shown in Fig. 2, when apparent motions appear in the CEST data, our method demonstrates improved quantification performance for amide proton transfer weighted (APTw) CEST using the asymmetry analysis (MTRasym). Compared with the PCA method, our method shows similar results when the head motion is slight (≤2mm, like in case 1), however, our method works much better when the head motion is huge ( ≥ 5mm, like in case 2). As shown in Fig. 3, we analyzed the accumulated variance ratio of the principal components calculated at step 4 in Fig. 1. Compared with the PCA method, our method shows a much higher accumulated variance ratio when the component number is smaller than 6, indicating the better performance of our method for regressing out the motion parameters.Note that in our method, the number of principal components needs to be carefully considered. Different selections of that may lead to different results. In the present work, an adjusted threshold was used to determine these parameters.
Conclusion
Our preliminary results demonstrate that our method could reduce the noise efficiently and improve the quantification results in the conditions of either with slight head motion or with huge head motion, indicating that our methods may have the potentials to improve the quantification accuracy for APTw or other kinds of CEST signals.Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (81873893), the Shanghai Science and Technology Committee (20ZR1407800),Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), ZJLab, and Shanghai Center for Brain Science and Brain-Inspired Technology.References
- Van Zijl P C M, Yadav N N. Chemical exchange saturation transfer (CEST): what is in a name and what isn't?[J]. Magnetic resonance in medicine, 2011, 65(4): 927-948.
- Zhang Y, Heo H Y, Lee D H, et al. Selecting the reference image for registration of CEST series[J]. Journal of Magnetic Resonance Imaging, 2016, 43(3): 756-761.
- Wech T, Köstler H. Robust motion correction in CEST imaging exploiting low‐rank approximation of the z‐spectrum[J]. Magnetic resonance in medicine, 2018, 80(5): 1979-1988.
- Breitling J, Deshmane A, Goerke S, et al. Adaptive denoising for chemical exchange saturation transfer MR imaging[J]. NMR in Biomedicine, 2019, 32(11): e4133.
DOI: https://doi.org/10.58530/2022/3835