3833
Compressed sensing chemical exchange saturation transfer for simultaneous APT, ssMT, and rNOE imaging1Siemens Healthineers Ltd., Shanghai, China, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, China, 4Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 5Human Phenome Institute, Fudan University, Shanghai, China
Synopsis
We proposed a fast 3D CEST imaging method using compressed sensing SPACE and demonstrated its routine clinical application for simultaneous APT, ssMT, and rNOE imaging. The total scan time of CEST images with 58 z-spectra and an off-resonance map was 6 minutes and 49 seconds. We used low refocusing flip angle and high bandwidth to achieve a narrow point spread function, and the images could be reproduced between different scans. This method could be used for tumor grading and treatment response assessment at 3T.
Introduction
Chemical exchange saturation transfer (CEST) is a molecular imaging method that can detect biomolecules in vivo via proton exchange between solute and water. Amide proton transfer (APT) imaging is a type of CEST imaging that has been used to grade tumors1, estimate ischemic penumbra2, and identify characteristics of tumors3. The most widespread APT imaging method quantifies the presence of amide by magnetization transfer ratio asymmetry (MTRasym) between +/-3.5 ppm of the z-spectrum. However, this method will also capture the contribution from the relayed nuclear Overhauser effect (rNOE) and asymmetric semi-solid magnetization transfer (ssMT). Although pre-saturation with equivalent B1 power around 2 µT reduces the rNOE contribution4, separately estimating these contributions provides additional diagnostic information3,5. A protocol optimized for a routine clinical use employing snapshot CEST and dense sampling of the z-spectrum was proposed at 3T6. However, the CEST contrast built by pre-saturation gradually decreases or increases to a steady state during readouts of gradient echoes in snapshot CEST. This results in more serious blurring along with partition encoding directions and varying point spread functions at different z-spectra. To overcome these drawbacks, we proposed a compressed sensing (CS) sampled SPACE CEST7 imaging method that simultaneously acquired B0-corrected APT, rNOE, and ssMT images within 7 minutes.Methods
We used a single-shot 3D CS SPACE sequence to acquire images for CEST imaging (TR = 3000 ms, TE = 16 ms, ETL = 260, bandwidth per pixel= 1000 Hz/px, flip angle = 60o, averages = 2, resolution = 2 x 2 x 4 mm3, matrix size= 96 x 96 x 12), with pre-saturation RF pulses (23 x 90 ms Gaussian pulses, 10 ms gap, mean B1= 0.7μT, 58 RF offsets8). We used two averages to reduce FID artifact. The total acquisition time was 5 mins and 50 secs. We acquired coil sensitivity images using the same imaging parameters without CS (acquisition time = 30 seconds). The off-resonance map was estimated by the phase evolution rate of multi-echo gradient-echo images (TR = 150 ms, TE = 4.92/7.38 ms, resolution = 2 x 2 x 4 mm3, matrix size= 96 x 96 x 12, scan time: 29 s). In summary, the total acquisition time for CEST, coil sensitivity determination, and off-resonance map was 6 mins and 49s. We used the 4 pools Lorentzian8 model of direct water saturation (DS), APT, ssMR, and rNOE to fit the measured data and estimate their contributions. A healthy volunteer, a meningioma patient, and a glioma patient were scanned on a 3T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany).Results
Figures 1A and B show the APT, ssMT, and rNOE images of the healthy volunteer of the first and the second scan, respectively. The similar images between the two scans demonstrate the reproducibility of our method. Because of the implementation of a low refocusing flip angle and high readout bandwidth, there was no visible blurring across the slices. Figure 1C shows the sampled z-spectra, Lorentzian-fitted lines for total signal, APT, ssMT, and rNOE of a voxel in the white matter. The 4-pool Lorentzian model provides a good fitting to the sampled data. Figure 2 shows the T1 weighted, APT, ssMT, and rNOE images of the meningioma patient. The CEST images depict a sharp contrast transition across lesion and structure boundaries. Figure 3 shows the APT images from the meningioma patient and the glioma patient. The glioma showed a stronger APT value than the meningioma.Discussion
Using CS SPACE CEST, we acquired a z-spectrum of a FOV that can cover most tumors within 6 seconds. The total scan time of 58 z-spectra, a coil sensitivity map, and an off-resonance map was 6 minutes and 49 seconds, which was suitable for routine clinical practice. The APT, ssMT, and rNOE images were reproducible across different scans. Compared to a previous study9, we used a higher bandwidth and lower flip angle to narrow the point spread function, and the images depicted sharp boundaries between the tumor and brain tissue (Figure 2). The APT value at 3T was higher than at 7T, and this was consistent with the literature8. We hypothesized that these signals may have come from amine groups due to smaller resonance frequency differences at 3T. Downfield suppressed APT and rNOE images were used for estimating histopathological characteristics and a treatment response assessment at 7T. It will be interesting to perform similar studies at 3T. We did not implement B1 correction in this study, but this could be done with one-point B1 correction when a B1 map is available.Conclusion
The proposed CS SPACE CEST was found to be a reproducible method for simultaneous APT, rNOE, and ssMT imaging using a clinically acceptable scan time (6 minutes and 49 seconds) at 3T.Acknowledgements
No acknowledgement found.References
1. Zaric, Olgica, et al. 7T CEST MRI: A potential imaging tool for the assessment of tumor grade and cell proliferation in breast cancer. Magnetic resonance imaging 59 (2019): 77-87.
2. Msayib, Y., et al. Quantitative CEST imaging of amide proton transfer in acute ischaemic stroke. NeuroImage: Clinical 23 (2019): 101833.
3. Paech, Daniel, et al. Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro-oncology 20.12 (2018): 1661-1671.
4. Zhou, Jinyuan, et al. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magnetic Resonance in Medicine 60 (2008): 842-849.
5. Meissner, Jan-Eric, et al. Early Response Assessment of Glioma Patients to Definitive Chemoradiotherapy Using Chemical Exchange Saturation Transfer Imaging at 7 T. Journal of Magnetic Resonance Imaging 50 (2019): 1268–77.
6. Goerke, Steffen, et al. Clinical Routine Acquisition Protocol for 3D Relaxation-Compensated APT and RNOE CEST-MRI of the Human Brain at 3T. Magnetic Resonance in Medicine 86 (2021): 393–404.
7. Ying-Hua Chu, et al. Highly accelerated compressed sensing chemical exchange saturation transfer. ISMRM 2021: 1826
8. Goerke, Steffen, et al. Relaxation-Compensated APT and RNOE CEST-MRI of Human Brain Tumors at 3 T. Magnetic Resonance in Medicine 82 (2019): 622–632.
9. Zhang, Yi, et al. Whole-Brain Chemical Exchange Saturation Transfer Imaging with Optimized Turbo Spin Echo Readout. Magnetic Resonance in Medicine 84 (2020): 1161–1172
Figures
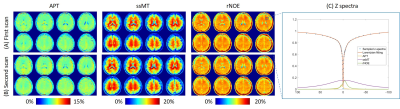
Figure 1. (A) and (B) Two different scans of APT, ssMT, and rNOE images of a healthy volunteer. (C) The measured spectra and the Lorentzian-fitted line of the spectra of one voxel in the white matter. The signal contribution from APT, ssMT, and rNOE is plotted.
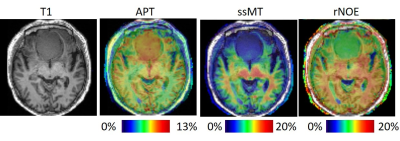
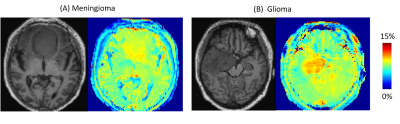
Figure 3. (A) T1 and APT image of the meningioma patient. (B) T1 and APT image of the glioma patient. The glioma shows a higher APT value.