3801
Motion correction of high-resolution spatiotemporally encoded MRI based on deep learning1Xiamen University, Xiamen, China, 2MSC Clinical & Technical Solutions, Philips Healthcare, Xiamen, China
Synopsis
In multi-shot high-resolution MRI, the motion of patients often leads to serious degradation of imaging quality. In this study, a novel motion correction method based on deep learning and spatiotemporally encoded MRI was proposed to address this problem. The proposed method is robust to motion without utilizing extra scan or parallel reconstruction. The results of simulation and in vivo rat brain experiments demonstrate its efficacy in reducing image motion artifacts when subject movement exists.
Introduction
High-resolution magnetic resonance image (MRI) exhibits increased significance in clinical diagnosis and scientific research, but multiple scans are required in order to complete the acquisition.1,2 The long scanning duration would inevitably make patients being restricted in a semi-closed environment more likely to move.3 On account of displacement and phase difference caused by motion between scans, the image quality is significantly deteriorated with undesirable motion artifacts.4 Spatiotemporally encoded (SPEN) MRI is a novel MRI method, which excites spin and rasterizes image contour in a spatial order.5 This special encoding strategy frees it from Fourier transform oriented reconstruction, but just adopts super-resolution (SR) reconstruction specifically related to spatial position, indicating that undersampling along the SPEN dimension will not produce image folding.6,7 In consideration of such merit, we propose a novel motion correction method based on SPEN MRI and the excellent fitting capability of deep network.Methods
Figure 1A presents the SPEN sequence we utilized, which differs from EPI in the excitation pulse. The chirp pulse induces quadratic phase, so that the SPEN signal contains mostly low-frequency information from the corresponding position. Meanwhile, high-frequency information from other positions approximately cancels each other for the violent oscillations of phase. Therefore, SPEN MRI will not be limited by Nyquist theorem, providing the theoretical basis for us to implement motion correction.As shown in Figure 1B, for each shot, SPEN enables the reconstruction of a low-resolution image without folding, which is difficult to achieve in EPI. Because the signal of EPI contains high-frequency or low-frequency information of all positions, which leads to serious artifacts during the reconstruction of a single shot utilizing limited information. Similar to PROPELLER sequence, low resolution SPEN images can in principle form a high-resolution image after registration, phase correction and combination. These procedures are conducted by deep network proposed in our method.
Figure 2 illustrates our process of generating simulation data as training datasets. We utilized MRiLab8 as the simulation tool to obtain SPEN signals with (red arrow) and without motion (blue arrow). Translation within ten voxels and rotation within 20 degrees were randomly applied for each acquisition. After one-dimensional Fourier transform of the frequency dimension and SR reconstruction of the SPEN dimension, we obtained the high-resolution image with and without motion. The phase differences between odd and even rows of the moving image were also added to mimic eddy current effect in real experiments. The moving images derived therein were fed into the network as inputs, while the still images were used as labels of the network.
Results
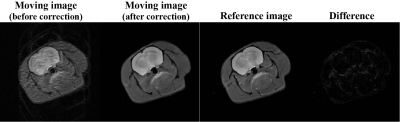
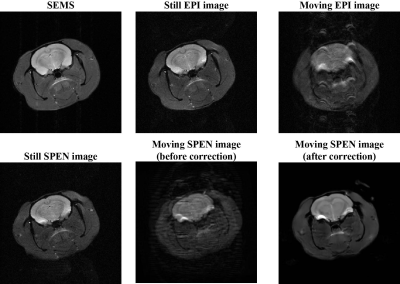
Figure 3 shows the numerical simulation results of rat brain with rigid motion. Motion is added to the simulation so that the final image is the superposition of motion states for all acquisitions. Prior to motion correction, the image quality deteriorated significantly in the existence of motion, showing obvious motion artifacts and texture blur. The distorted image is well restored and motion artifacts are roughly eliminated post to the correction. According to the difference map of reference and corrected images, we can see that the corrected image closely approached to the reference image. The loss of some tiny image details was observed, this can be explained as that the spatial motion may lead to repeated acquisition at some regions while missed acquisition at other regions.Figure 4 illustrates the results of in vivo rat brain experiments. Rat images collected in the presence of minimum movement are regarded as still images. Among them, both still EPI and SPEN images showed similar performances with pretty good image quality. However, the SPEN and EPI images are obviously affected by motion artifacts in moving scenarios, of which the former can be effectively corrected with results highly resembled the still SPEN image whereas it would be difficult to correct the moving EPI image without the help of extra scan or parallel reconstruction.
Discussion and conclusion
The proposed SPEN and deep-learning based MRI motion correction method exhibits great resistance against motion artifacts in both simulation and in vivo rat brain experiments. Our approach has enhanced the robustness of scanning to motion, making subjects not have to strictly kept motionless. In the future, it is worth studying to extending this motion correction method to non-rigid motion.Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant numbers 82071913 and 11775184, and Science and Technology Project of Fujian Province 2019Y0001. We also thank postdoctoral fellow Lina Xu for polishing the article.References
1. Jeong H-K, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot sense EPI at 7 T. Magn Reson Med. 2013;69:793–802.
2. Guhaniyogi S, Chu M-L, Chang H-C, Song AW, Chen N-K. Motion immune diffusion imaging using augmented muse for high-resolution multi-shot EPI. Magn Reson Med. 2016;63:639–652.
3. Pipe JG. Motion correction with PROPELLER MRI: Application to head motion and free-breathing cardiac imaging. Magn Reson Med. 1999;42(5):963-969.
4. Skare S, Newbould RD, Clayton DB, Bammer R. PROPELLER EPI in the other direction. Magn Reson Med. 2006;55(6):1298-1307.
5. Tal A, Frydman L. Single-scan multidimensional magnetic resonance. Prog Nucl Magn Reson Spectrosc. 2010;57(3):241-292.
6. Cai CB, Dong JY, Cai SH, et al. An efficient de-convolution reconstruction method for spatiotemporal-encoding single-scan 2D MRI. J Magn Reson. 2013;228:136-147.
7. Cousin SF, Liberman G, Solomon E, Otikovs M, Frydman L. A regularized reconstruction pipeline for high-definition diffusion MRI in challenging regions incorporating a per-shot image correction. Magn Reson Med. 2019;82(4):1322-1330.
8. Liu F, Velikina JV, Block WF, Kijowski R, Samsonov AA. Fast realistic MRI simulations based on generalized multi-pool exchange tissue model. IEEE Trans Med Imaging. 2017;36(2):527-537.
Figures
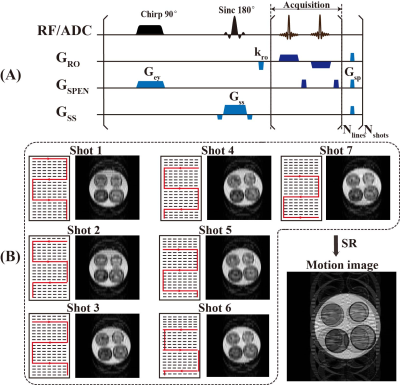
Figure 1. (A) Multi-shot SPEN sequence. (B) The low-resolution image reconstructed from one shot and the high-resolution image with motion artifacts generated by the combination of all shots.
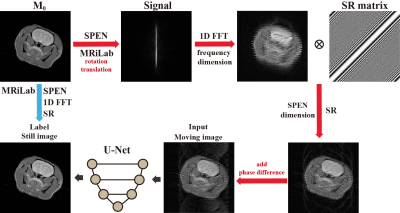
Figure 2. Flow chart of generation of simulated training dataset.