3797
Basal Ganglia Connectivity as Assessed by Resting-State fMRI in Genetic Parkinson’s Disease1Aysel Sabuncu Brain Research Center-UMRAM, Ankara, Turkey, 2Department of Nuclear Medicine, Hacettepe University, Ankara, Turkey, 3Department of Neurology, Hacettepe University, Ankara, Turkey, 4Department of Radiology, Hacettepe University, Ankara, Turkey
Synopsis
Genetic Parkinson’s Disease (gPD) displays distinct clinical, demographic and pathological features from idiopathic Parkinson’s Disease (PD). The present study aims to search for alterations in the basal ganglia network (BGN) including substantia nigra (SN) and Subthalamic nucleus (STN) in a peculiar group of gPD using resting state (RS) fMRI. Genetic PD patients revealed less activation in the basal ganglia, SN+STN pathway and reduced connectivity with ACC, MCC and insula, which may reflect frontal lobe and autonomic dysfunction in these patients.
Introduction
Usually starting at younger ages and presenting with a slower clinical course, rare monogenic forms of Parkinson’s disease (gPD) including Parkin, SNCA, LRRK2, PINK1 and DJ-1 mutations remain unelucidated1. Underlying brain network changes may be responsible for the diversity of clinical symptomatology with motor, autonomic and cognitive components. Because loss of the dopaminergic neurons in the substantia nigra (SN) and degeneration of the striatonigral fibers are considered primary pathophysiologic mechanism, we focused on the BGN including basal ganglia structures in addition to SN and subthalamic nucleus (STN) using resting-state (RS) fMRI in this peculiar patient group.Methods
IRB approved this study and all participants gave informed consent. Nine gPD patients and 13 healthy controls (HCs) took part in this study. All participants had normal brain MRI as evaluated by an experienced neuroradiologist. MDS-Unified Parkinson's Disease Rating Scale (MDS-UPDRS)2 and Mini Mental State Examination (MMSE) were obtained from patients. All subjects had RS-fMRI on a 3.0-T scanner magnet equipped with a 32 channel phase array head coil. A Gre EPI sequence (TR/TE, 1000/25 ms; 164 volumes, with 3.3 mm slice thickness), 3D T1W MPRAGE and T2W axial images were obtained while the subjects were lying quietly inside the scanner. RS-fMRI data preprocessing and analyses were obtained with CONN toolbox3 implemented in Matlab20204. Preprocessing steps included motion correction using SPM125, realign & unwarp procedure6 where all images were coregistered and resampled to a reference first image in the session. Noise reduction was performed using the Component Based Noise Correction method. Temporal frequencies below 0.008 Hz or above 0.09 Hz are removed from the BOLD signal in order to focus on slow-frequency fluctuations while minimizing the influence of physiological, head-motion and other noise sources. Following functional and anatomical data normalization into standard MNI space and segmentation into CSF, grey and white matter, smoothing was applied with a 8 mm Gaussian kernel. SPM12 and xjview toolbox7 was used for MNI coordinates and anatomical labeling, and Wfupickatlas8 toolbox was used for ROI definition. Seed based connectivity (SBC) was applied to analyze the data to characterize the connectivity patterns with a pre-defined seed for BGN networks. SBC maps were computed as the Fisher-transformed bivariate correlation coefficients between an ROI BOLD time series and each individual voxel BOLD time series. Global BOLD signal change was calculated with t test along with a color scale representing the t value of the BOLD signal (voxel threshold p<.001 and cluster threshold p<.05, cluster size k>10).Results
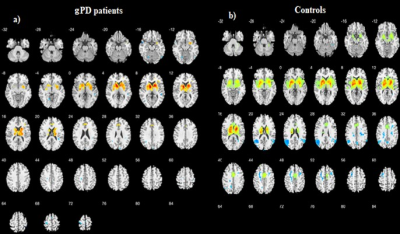
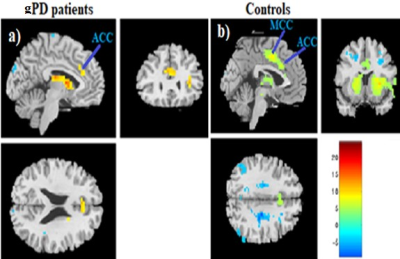
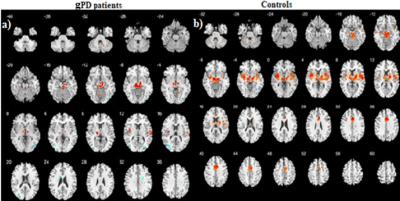
Patients and HCs were similar in terms of gender and age (p>0.05). Demographic and clinical characteristics of gPD and HC groups: gPD (n=9, Female/Male:4/5), Age (mean±SD: 39.66± 8.09, p=0.608,Unified Parkinson Disease Rating Scale (UPDRS): mean±SD 11.33±3.42, Mini Mental State Examination (MMSE): 27.87± 2.23), HCs (n=13, Female/Male:7/6), Age (mean±SD: 27.15± 4.02,p=0.701, UPDRS): not applicable (NA), MMSE: NA). SBC maps were obtained for two groups selecting caudate, putamen, globus pallidus (all referred as BG) and SN+STN bilaterally as a seed. Compared to HCs, gPD group showed a profound reduction in activation in the basal ganglia and SN, STN (Figure 1). Caudate nucleus showed the strongest correlation (r=0.74, t=16) in BGN and deactivations were observed in the motor area in gPD (Fig. 1a). Anterior cingulate cortex (ACC) clusters decreased remarkably and medial cingulate cortex (MCC) activation observed in HCs almost disappeared in gPD patients (Fig. 2a, b). Additional separate analysis of SN+STN revealed profoundly diminished activity in the basal ganglia and thalamus, insula, ACC and MCC bilaterally (z=24) in gPD than HCs (Fig. 3 a, b).Demographic and clinical characteristics of
Discussion
Genetic PD patients displayed diminished activation in the BG, thalamus and SN, STN when a seed of BG structures and SN,STN were used. Diminished activation was observed also in anterior and medial cingulate gyri, which may be related to executive functioning abnormalities in gPD9patients. Reduced insular connectivity may also reflect visceral and autonomic dysfunction in symptomatology10 (R). Number of participants due to COVID restrictions is the main limitation of this study, however researchers will be retrieving more subjects as the project is going on.Conclusion
We intend to expand our study with more gPD patients, also compare them with idiopathic PD patients to enhance our findings and investigate their clinical correlations.Acknowledgements
No acknowledgement found.References
1. Schapira A H, Jenner P. Etiology and pathogenesis of Parkinson's disease. Mov. Disord. 2011 May;26(6):1049-55. doi: 10.1002/mds.23732.
2. International Parkinson and Movement Disorder Society. MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). https://www.movementdisorders.org/MDS- Files1/Education/Rating-Scales/MDS-UPDRS_Turkish_OfficialTranslation_FINAL.pdf [11.03.2021].
3. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity, 2(3), 125-141).
4. Mathworks Inc., Natick MA, USA
5. https://www.fil.ion.ucl.ac.uk/spm/software/spm12/
6. Andersson JLR, Hutton C, Ashburner J, Turner R, Friston K. Modelling geometric deformations in EPI time series. NeuroImage 2001;(13), 90-919.
7. https://www.alivelearn.net/xjview
8. https://www.nitrc.org/projects/wfu_pickatlas/
9. Lewis SJG, Shine JM, Duffy S, Halliday G, Naismith SL. Anterior cingulate integrity: executive and neuropsychiatric features in Parkinson's disease. Movement disorders. 2012;27(10), 1262-1267.
10. Uddin QL, Nomi JS, Ghazirri J, Boucher O. Structure and Function of the Human Insula. J Clin. Neurophysiol. 2017 Jul;34(4):300-306.
Figures