3791
Differentiating metastatic from nonmetastatic lymph nodes in cervical cancer patients by IVIM-DWI and DKI1Department of Radiology, Yunnan Cancer Hospital, Third Affiliated Hospital of Kunming Medical University, Kunming, China, 2Clinical Science, Philips Healthcare, Chengdu, China
Synopsis
This study aimed to investigate the diagnostic value of intravoxel incoherent motion (IVIM) diffusion-weighted imaging and diffusion kurtosis imaging (DKI) in distinguishing metastatic from nonmetastatic lymph nodes (LNs) in cervical cancer patients. The clinical and imaging data of 102 patients with cervical cancer were collected prospectively, including 38 patients with LN metastasis and 64 patients without LN metastasis. The morphological parameters, diffusion parameters and tumor markers of primary tumors and LNs were measured and compared between the two groups. The results showed that the diagnostic efficiency of diffusion parameters were not as good as morphological parameters.
Introduction
Worldwide, cervical cancer is the fourth most common malignancy in women, with an extremely high morbidity and mortality rate, and it represents a serious threat to women’s health and life. Despite widespread screening programs and well-established treatment options, the long-term survival of cervical cancer patients has not been significantly improved due to pelvic lymphatic metastasis and other risk factors.Currently, CT and MRI are the main imaging modalities to evaluate LN metastasis of cervical cancers, but these methods are mainly based on an LN short axis>10 mm, which cannot provide accurate histopathological information and has low reliability. PET-CT can improve the diagnostic sensitivity, but it is expensive. Therefore, ways to improve the accuracy of the imaging diagnosis of metastatic LNs and imaging biomarkers of LN metastasis are urgently needed.
Diffusion-weighted imaging (DWI) can be used to probe the structure of biological tissue by measuring the diffusion of water molecules. The apparent diffusion coefficient (ADC) value of metastatic LNs is lower than that of normal LNs due to the limited diffusion of water molecules in proliferating tumor cells. However, the DWI identification threshold often overlaps and lacks absolute standards, representing significant limitations. With the development of magnetic resonance equipment and mathematical models, intravoxel incoherent motion (IVIM) imaging and diffusion kurtosis imaging (DKI) have emerged based on traditional DWI. IVIM can distinguish pure molecular diffusion from perfusion diffusion to evaluate the perfusion of living tissue without using contrast agents. DKI can describe the degree of deviation from the Gaussian distribution in water molecules, which can more accurately reflect the tumor microenvironment. Preliminary studies have shown that IVIM has a certain significance in the diagnosis of lymphatic metastasis in cervical cancers, but wide variations exist in the results of different studies. Moreover, only one report used DKI for the diagnosis of lymph node metastasis in cervical cancers. Therefore, this study aimed to explore the value of IVIM and DKI in the diagnosis of lymphatic metastasis in cervical cancers.
Material and Methods
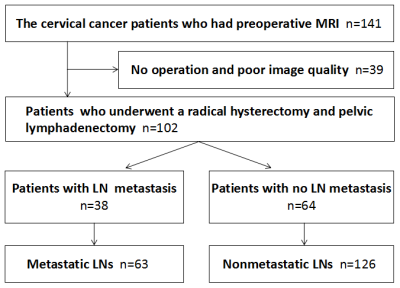
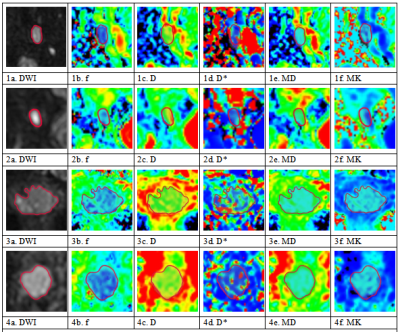
Patients In total, 102 patients were ultimately included in this study (mean age, 48.69 years; range, 20-72 years), including 38 patients with LN metastasis and 64 patients without LN metastasis (see Figure 1 for details).Imaging and data acquisition MR imaging was performed using a Philips 3.0 T scanner (Ingenia, 3.0 T; Philips Medical Systems, The Netherlands). All patients were scanned routinely with an 8-channel, phased-array body coil in the supine position. The parameters of IVIM and DKI were as follows. IVIM was performed with a free-breathing single-shot spin-echo echo-planar sequence in the coronal plane (repetition time/echo time: 9407 ms/60 ms; slice thickness/gap: 4 mm/1 mm; matrix: 128×128; field of view: 380 mm×380 mm; number of excitations: 1; 11 b-values: 0, 20, 50, 100, 150, 200, 500, 800, 1000, 1500 and 2000 s/mm2; and scan time: 12 min 14 s). DKI was performed with a free-breathing single-shot spin-echo echo-planar sequence using 15-directional motion-probing gradients in the coronal plane (repetition time/echo time: 2626 ms/100 ms; slice thickness/gap: 4 mm/1 mm; matrix: 128×128; field of view: 380 mm×380 mm; number of excitations: 2; 3 b-values: 0, 800 and 1500 s/mm2; and scan time: 6 min 33 s).Imaging data analysis and processingRoutine MRI images were analyzed by two pelvic radiologists with more than 5 years of experience with PACS workstations, and the following MR features were analyzed: tumor diameter, location, morphology, lesion margin, T1 and T2 signal intensity, DWI intensity and enhancement pattern. The DWI images were analyzed by using the Philips IntelliSpace Portal (Philips, Best, The Netherlands). The IVIM and DKI images were analyzed by using IMAgenGINE MRToolbox software (Vusion Tech Ltd). Combined with axial T2WI and enhanced scan sequence, the region of interest (ROI) was delineated on the largest slice of the primary tumor and LN, and all ROIs were delineated to avoid cysts and necrotic areas.
Correspondence between imaging and histopathology We separated the largest lymph node from each side of the pelvic lymph node specimen and sent it to the Department of Pathology for evaluation. Furthermore, we observed the largest lymph node on the same side on MRI to ensure that the lymph node observed on MRI corresponds one-to-one to the lymph node detected by histopathology.
Results
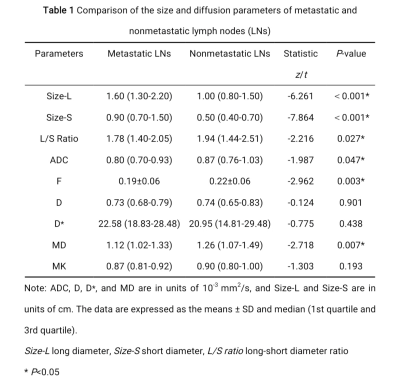
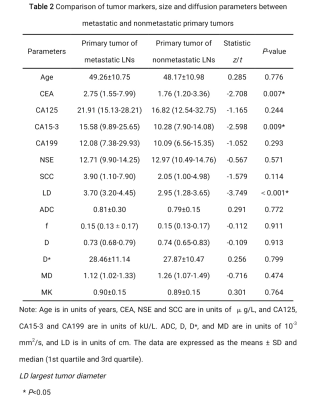
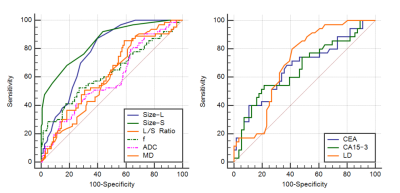
In total, 102 patients, including 38 patients pathologically shown to have lymphatic metastasis (containing 63 metastatic LNs) and 64 patients pathologically shown to have no lymphatic metastasis (containing 126 nonmetastatic LNs ) were included in this study. The metastatic LNs exhibited a lower apparent diffusion coefficient, mean diffusivity, and flowing blood volume fraction, a higher short and long diameter, and a lower long-short diameter ratio than those of nonmetastatic LNs. The largest tumor diameter, CEA and CA15-3 levels of the patients were higher in the metastatic groups compared with the nonmetastatic groups (P<0.05). The short diameter of LNs exhibited the highest diagnostic value, with an area under the curve of 0.848.Conclusion
IVIM-DWI and DKI can potentially noninvasively obtain information to evaluate the presence of LN metastasis in cervical cancer. However, traditional morphological parameters of LNs still has a better diagnostic efficiency.Acknowledgements
No acknowledgement found.References
1. Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249.
2. Shen WC, Chen SW, Liang JA, Hsieh TC, Yen KY, Kao CH (2017) [18]Fluorodeoxyglucose Positron Emission Tomography for the Textural Features of Cervical Cancer Associated with Lymph Node Metastasis and Histological Type. Eur J Nucl Med Mol Imaging 44:1721-1731.
3. Kidd EA, Siegel BA, Dehdashti F et al (2010) Lymph Node Staging by Positron Emission Tomography in Cervical Cancer: Relationship to Prognosis. J Clin Oncol 28:2108-2113.
4. Twu NF, Ou YC, Liao CI et al (2016) Prognostic factors and adjuvant therapy on survival in early-stage cervical adenocarcinoma/adenosquamous carcinoma after primary radical surgery: A Taiwanese Gynecologic Oncology Group (TGOG) study. Surg Oncol 25:229-235.
5. Pma J, Fl B, Cuck L et al (2021) Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentre randomised trial (SENTICOL-2). Eur J Cancer 148:307-315.
6. Brar H, Hogen L, Covens A (2017) Cost-effectiveness of sentinel node biopsy and pathological ultrastaging in patients with early-stage cervical cancer. Cancer 123:1751-1759.
7. Salvo G, Ramirez PT, Levenback CF et al (2017) Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol Oncol 145:96-101.
8. Michaan N, Laskov I, Aizic A, Brautbar O, Dan G (2021) Laparoscopic sentinel lymph node dissection followed by open radical hysterectomy for early stage cervical cancer: A pilot study. Int J Gynaecol Obstet 152:183-187.
9. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R (2018) Cancer of the cervix uteri. Int J Gynaecol Obstet 143:22-36.
10. Chu HK, Park H, Lee HY et al (2020) Computed Tomography Radiomics for Residual Positron Emission Tomography-Computed Tomography Uptake in Lymph Nodes after Treatment. Cancers (Basel) 12:3564.
11. Jung W, Park KR, Lee KJ et al (2017) Value of imaging study in predicting pelvic lymph node metastases of uterine cervical cancer. Radiat Oncol J 35:340-348.
12. Brunette LL, Bonyadlou S, Ji L et al (2018) Predictive Value of FDG PET/CT to Detect Lymph Node Metastases in Cervical Cancer. Clin Nucl Med 43:793-801
13. Shen G, Zhou H, Jia Z, Deng H (2015) Diagnostic performance of diffusion-weighted MRI for detection of pelvic metastatic lymph nodes in patients with cervical cancer: a systematic review and meta-analysis. Br J Radiol 88:20150063.
14. Song J, Hu Q, Huang J, Ma Z, Chen T (2018) Combining tumor size and diffusion-weighted imaging to diagnose normal-sized metastatic pelvic lymph nodes in cervical cancers. Acta Radiol 60:388-395.
15. Wu Q, Zheng D, Shi L, Liu M, Wang M, Shi D (2017) Differentiating metastatic from nonmetastatic lymph nodes in cervical cancer patients using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging. Eur Radiol 27:5272-5279.
16. Meng N, Wang X, Sun J et al (2020) Application of the amide proton transfer-weighted imaging and diffusion kurtosis imaging in the study of cervical cancer. Eur Radiol 30:5758-5767.
17. Xu C, Du S, Zhang S, Wang B, Dong C, Sun H (2020) Value of integrated PET-IVIM MR in assessing metastases in hypermetabolic pelvic lymph nodes in cervical cancer: a multi-parameter study 30:2483-2492.
18. Yamada I, Oshima N, Wakana K et al (2021) Uterine Cervical Carcinoma: Evaluation Using Non-Gaussian Diffusion Kurtosis Imaging and Its Correlation With Histopathological Findings. J Comput Assist Tomogr 45:29-36.
19. Liu Y, Liu H, Bai X, Ye Z, Sun H, Bai R et al (2011) Differentiation of metastatic from non-metastatic lymph nodes in patients with uterine cervical cancer using diffusion-weighted imaging. Gynecol Oncol 122:19-24.
20. Park SO, Kim JK, Kim KA et al (2009) Relative apparent diffusion coefficient: Determination of reference site and validation of benefit for detecting metastatic lymph nodes in uterine cervical cancer. J Magn Reson Imaging 29:383-390.
21. Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR (2010) An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med 64:1580-1585.
22. Lin G, Ho KC, Wang JJ et al (2008) Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging 28:128-135.
23. Nakai G, Matsuki M, Inada Y et al (2008) Detection and evaluation of pelvic lymph nodes in patients with gynecologic malignancies using body diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr 32:764-768.
24. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497-505.
25. Zhang SX, Jia QJ, Zhang ZP et al (2014) Intravoxel incoherent motion MRI: emerging applications for nasopharyngeal carcinoma at the primary site. Eur Radiol 24:1998-2004.
26. Andreou A, Koh DM, Collins DJ et al (2013) Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol 23:428-34.
27. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432-1440.
28. Turan T, Yildirim BA, Tulunay G, Boran N, Kose MF (2010) Prognostic effect of different cut-off values (20mm, 30mm and 40mm) for clinical tumor size in FIGO stage IB cervical cancer. Surg Oncol 19:106-113.
29. Pallavi VR, Devi KU, Mukherjee G, Ramesh C, Bafna UD (2012) Relationship between lymph node metastases and histopathological parameters in carcinoma cervix: A multivariate analysis. J Obstet Gynaecol 32:78-80.
30. Xu C, Li X, Shi Y, Wang B, Sun H (2020) Combinative evaluation of primary tumor and lymph nodes to predict pelvic lymphatic metastasis in cervical cancer: an integrated PET-IVIM MRI study. Cancer Imaging 20:21.
31. Huang EY, Huang YJ, Chanchien CC et al (2012) Pretreatment carcinoembryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following definitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervix. Radiat Oncol 78:13.
32. Ikeda S, Yoshimura K, Onda T et al (2012) Combination of squamous cell carcinoma-antigen, carcinoembryonic antigen, and carbohydrate antigen 19-9 predicts positive pelvic lymph nodes and parametrial involvement in early stage squamous cell carcinoma of the uterine cervix. J Obstet Gynaecol Res 38:1260-1265.
33. Wu SG, He ZY, Ren HY et al (2016) Use of CEA and CA15-3 to Predict Axillary Lymph Node Metastasis in Patients with Breast Cancer. J Cancer 7:37-41.
34. Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP (2016) Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 57:327-334.
35. Duffy MJ, Evoy D, Mcdermott EW (2010) CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta 411:1869-1874.
36. Guo G, Wang Y, Zhou Y et al (2019) Immune cell concentrations among the primary tumor microenvironment in colorectal cancer patients predicted by clinicopathologic characteristics and blood indexes. J Immunother Cancer 7:179.
37. Xu D, Wang D, Wang S, Tian Y, Long Z, Ren X (2017) Correlation Between Squamous Cell Carcinoma Antigen Level and the Clinicopathological Features of Early-Stage Cervical Squamous Cell Carcinoma and the Predictive Value of Squamous Cell Carcinoma Antigen Combined With Computed Tomography Scan for Lymph Node Metastasis. Int J Gynecol Cancer 27:1935-1942.
38. Zhou Z, Li W, Zhang F, Hu K (2017) The value of squamous cell carcinoma antigen (SCCa) to determine the lymph nodal metastasis in cervical cancer: A meta-analysis and literature review. PLoS One 12:e0186165.
Figures