3790
Sex-dependent Pathological Aging Effect on Caudate Functional Connectivity in Mild Cognitive Impairment1Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, United States, 2University of Nevada Las Vegas, Las Vegas, NV, United States, 3University of Colorado, Boulder, CO, United States
Synopsis
Resting-state fMRI was used to investigate the aging effect on caudate function in mild cognitively impaired participants, with the focus of sex-dependent effect. Graph theory analysis was conducted to derive caudate nodal strength, which was then input to linear mixed effect model to evaluate its association with age. A striking aging effect was observed only in women with MCI but not in men with MCI, which was closely related to cognitive decline in woman participants. This finding suggests that caudate may be critical for alleviating cognitive decline in women with MCI.
INTRODUCTION
Brain aging is characterized by considerable heterogeneity, including the differences between regions and the variability induced by demographic factors and symptomatic or presymptomatic pathology. Mild cognitive impairment (MCI) is known to be a heterogeneous condition [1]. While episodic memories are established and maintained by an interplay between the medial temporal lobe and other cortical regions, the aging-related degradation of the frontostriatal system is suggested to be a driving factor of episodic memory decline in older adults [2]. Furthermore, emerging evidence suggests that women differ from men in multiple neurological aspects, including brain function, cognitive domains, cognitive decline [3, 4]. In this study, we focused on investigating aging effect on caudate, one important region in frontostriatal system, among mild cognitive impairment (MCI) participants using resting-state functional magnetic resonance (fMRI) data and evaluating the role of sex in the process.METHODS
277 functional magnetic resonance imaging (fMRI) sessions from 163 cognitive normal (CN) older adults and 309 sessions from 139 participants with MCI were included as the main sample in our analysis. Pearson’s correlation was used to characterize the functional connectivity (FC) between caudate and each brain region in the AAL atlas (94 regions of interests). Weighted FC networks were used in the graph theoretical analysis to derive the caudate nodal strength, which characterizes the overall connectivity strength of all regions with caudate. Association analysis between caudate nodal strength and age was carried out in MCI and CN separately using linear mixed effect (LME) model with both women and men included (education, handedness, sex, Apolipoprotein E4 and intra-subject effect as covariates). LME model was then applied to women and men separately within MCI group to evaluate aging effects on caudate nodal strength. Similar association analysis was conducted between age and individual region’s connectivity with caudate to unveil which region’s connectivity with caudate substantially contributes to aging effect on caudate nodal strength. With the fMRI subset having amyloid positron emission tomography (PET) scans available in the same visits, the role of amyloid burden in the association of age with caudate nodal strength was examined using analysis of covariance (ANCOVA). Furthermore, the pair-wise correlation analysis of cognitive scores with age and caudate nodal strength was conducted. The same statistical analysis with an independent fMRI dataset acquired with fast fMRI acquisition protocol was used to further validate the sex-dependent aging effects observed in the main sample.RESULTS
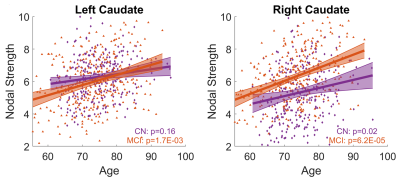
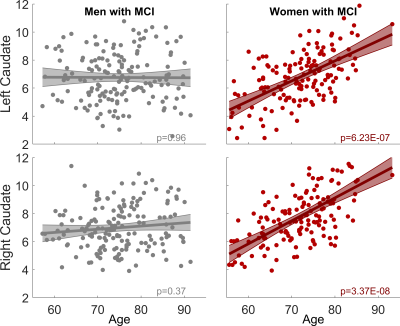
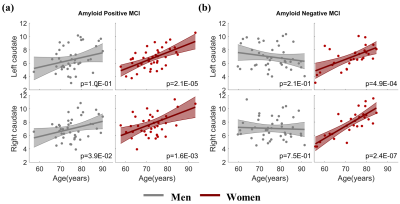
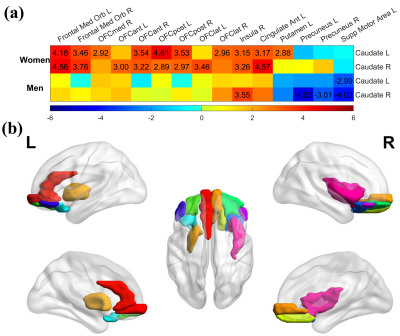
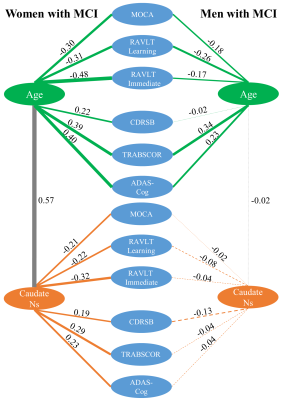
With both women and men included in the analysis, the MCI group had significantly stronger age-related increase of caudate nodal strength (Left: p = 0.0017; right: p = 6.2x10-5, see Figure 1) than the CN group (Left: p = 0.16; right: p = 0.02, see Figure 1). Analyzing women and men separately revealed that the aging effect on caudate nodal strength among MCI participants was significant only for women (Figure 2, left: P=6.23x10-7, right: P=3.37x10-8), but not for men (Figure 2, P>0.3 for bilateral caudate). Similar sex-dependent aging effects on caudate nodal strength were observed with the independent sample. ANCOVA showed that the aging effects on caudate nodal strength were not significantly mediated by brain amyloid burden. Sex-dependent aging effects on caudate nodal strength were consistently observed in both amyloid positive and amyloid negative MCI participants (Figure 3). Caudate connectivity with ventral prefrontal cortex substantially contributed to the aging effect on caudate nodal strength in women with MCI (Figure 4). While older age was associated with worse cognition for both women and men with MCI, higher caudate nodal strength was significantly associated with worse cognition in women but not in men with MCI (Figure 5).DISCUSSION
While age-related change in the caudate is recognized as an important factor to predict cognitive decline over the life span [5], compared to the medial temporal lobe system, far less attention has been paid to the involvement of the caudate in dementia, and most of these prior MRI studies focused on volumetric changes of caudate with diverse conclusions [6-8]. Our analysis revealed a striking aging effects on caudate nodal strength in women with MCI but not in men with MCI. In addition, we showed that caudate was closely related to cognitive decline in women with MCI, suggesting that caudate may be sensitive to the pathology experienced in the woman participants. A previous fMRI study showed differing brain functional alteration between MCI and CN in women and men, based on multiple global network metrics [9]. These observations together suggest that the alteration of brain function in MCI is sex-dependent.CONCLUSION
Sex modulates the pathological aging effects on caudate in MCI regardless of amyloid status. Caudate nodal strength may be a sensitive biomarker of pathological aging in women with MCI. Sex could be a critical factor for developing personalized medication strategy to alleviate cognitive decline in MCI.Acknowledgements
This research project was supported by the NIH (Grant No. 1RF1AG071566, COBRE 5P20GM109025 and NeVADRC; P20-AG068053), Cleveland Clinic Keep Memory Alive Young Investigator Award, The Women's Alzheimer's Movement, a private grant from Stacie and Chuck Matthewson, a private grant from Peter and Angela Dal Pezzo, and a private grant from Lynn and William Weidner. Part of the data collection and sharing for this study was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson &Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.References
1. Lambon Ralph, M.A., et al., Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer’s disease: a cross‐sectional and longitudinal study of 55 cases. Brain, 2003. 126(11): p. 2350-2362.
2. Fjell, A.M., et al., Brain Events Underlying Episodic Memory Changes in Aging: A Longitudinal Investigation of Structural and Functional Connectivity. Cereb Cortex, 2016. 26(3): p. 1272-1286.
3. Li, R. and M. Singh, Sex differences in cognitive impairment and Alzheimer’s disease. Frontiers in neuroendocrinology, 2014. 35(3): p. 385-403.
4. Holland, D., et al., Higher rates of decline for women and apolipoprotein E ε4 carriers. American Journal of Neuroradiology, 2013. 34(12): p. 2287-2293.
5. Greven, C.U., et al., Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA psychiatry, 2015. 72(5): p. 490-499. 6. Ryan, N.S., et al., Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease. Brain, 2013. 136(5): p. 1399-1414.
7. Barber, R., et al., Volumetric MRI study of the caudate nucleus in patients with dementia with Lewy bodies, Alzheimer's disease, and vascular dementia. Journal of Neurology, Neurosurgery & Psychiatry, 2002. 72(3): p. 406-407.
8. Persson, K., et al., Finding of increased caudate nucleus in patients with Alzheimer's disease. Acta Neurologica Scandinavica, 2018. 137(2): p. 224-232. 9. Cieri, F., et al., Sex Differences of Brain Functional Topography Revealed in Normal Aging and Alzheimer’s Disease Cohort. Journal of Alzheimer's Disease, 2021. 80: p. 979-984.
Figures