3786
Increased Vascular Permeability of 17O-labeled Water in the Amyotrophic Lateral Sclerosis Model Mice using Indirect Proton MRI1Department of Diagnostic and Interventional Radiology, Hokkaido University Hospital, Sapporo, Japan, 2Faculty of Dental Medicine, Department of Radiology, Hokkaido University, Sapporo, Japan, 3Live Imaging Center, Central Institute for Experimental Animals, Kawasaki, Japan, 4Department of Diagnostic Imaging, Hokkaido University Graduate School of Medicine, Sapporo, Japan, 5Global Center for Biomedical Science and Engineering, Hokkaido University Faculty of Medicine, Hokkaido University, Sapporo, Japan
Synopsis
We evaluated the increased vascular permeability of 17O-labeled Water in the amyotrophic lateral sclerosis (ALS) mice using indirect proton MRI. The spinal cord of nine ALS mice and ten wild-type (WT) mice were scanned with 7T MRI with the T2-weighted RARE sequence. In the femoral vein, 17O-labeled water was intravenously injected. In ROIs of the spinal cord, the 17O concentration increased more in ALS mice than in the control mice. This latest method can detect the abnormalities in water kinetics probably caused by the impairments in blood–spinal cord barrier of ALS mice.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease involving the lower and upper motor neurons. The underlying pathogenic mechanism of the disease is not clear, and there are no sensitive biomarkers for early-stage detection of ALS. The impairment of blood–spinal cord barrier (BSB) is a key factor aggravating the motor neuron damage [1, 2].Indirect proton magnetic resonance imaging (MRI) using 17O-labeled water as the contrast agent, can analyze water kinetics, including the water-channel function, in several disorders [3, 4]. Indirect proton MRI of 17O-labeled water can be applied to clinical MRI scanners without the need for special equipment. In this study, we hypothesized that indirect proton MRI, using 17O-labeled tracers, can detect impairments in the BSB and increased vascular permeability and can be a biomarker for early-stage ALS detection.
MATERIALS AND METHODS
All experiments were approved by the Animal Study Committee of the Central Institute for Experimental Animals in Japan. ALS mice (n = 9; B6.B-Vps54wr/J1 [5], the Jackson Laboratory) and wild-type (WT) mice (n = 10) were scanned using a 7T Biospec 70/16 MRI scanner (Bruker biospec GmbH; Ettlingen, Germany) equipped with gradient systems with a maximum strength of 700 mT/m. Two-dimensional T2-weighted axial images were serially acquired with the following parameters: effective TE, 60 ms; TR, 1000 ms; FOV, 12.8 × 12.8 mm; matrix, 128 × 128; slice thickness, 1.5 mm; number of excitations, 1; number of echoes in Rapid Acquisition with Refocusing Echoes, 8; single scan time, 13 s; number of repetitions, 50; total scan time, 10 min 50 s. In the femoral vein, 20mol% 17O-labeled water (5 ml/kg) was rapidly injected 2 minutes after the commencement of the MRI scan. Image analysis of the data obtained was performed using in-house software (Perfusion Mismatch Analyzer: PMA) [6]. The 17O concentration in the region of interest (ROI) was calculated based on the signal intensity [3, 4].RESULTS and DISCUSSION
Figure 1 shows the source images on which the ROIs were manually placed. Figure 2 shows the dynamic curves of the 17O concentration in the (a) whole spinal cord, (b) gray matter, and (c) lateral cord. The 17O concentration in the spinal cord, gray matter, and lateral cord, was observed to reach its peak value, approximately 50 s after the injections in both the groups. The value was observed to be higher in the ASL mice (test group) compared to the WT mice (control group), with a statistically significant difference in the observed values. These results suggest the presence of abnormalities in water kinetics and water space, resulting from impairments in BSB, in ALS model mice.There were no significant differences in the baseline intensity of lateral cord on T2-weighted images between the two groups (Figure 2d). High signal intensity, on T2-weighted images of the lateral corticospinal tract, reflecting degeneration, is a key finding in ALS [7]. Thus, abnormalities in water kinetics on indirect proton MRI of 17O-labeled water can be a biomarker for early ALS in patients who do not exhibit spinal cord abnormalities on conventional MRI. The limitations of this study are the inadequate signal-to-noise ratio, low sensitivity of indirect proton MRI in small animals, and lack of comparison with gadolinium-enhanced MRI.
CONCLUSION
Indirect proton MRI of 17O-labeled water can detect the abnormalities in water kinetics probably caused by the impairments in BSB of ALS model mice. It may well have pertinence in evaluating the impairments in BSB and increased vascular permeability.Acknowledgements
No acknowledgment found.References
[1] Garbuzova-Davis S et al., Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 2012; 1469: 114–128.
[2] Miyazaki K et al., Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res. 2011; 89(5): 718–728.
[3] Kudo K et al., Indirect Proton MR Imaging and Kinetic Analysis of 17O-Labeled Water Tracer in the Brain. Magn Reson Med Sci. 2018; 17(3): 223–230.
[4] Kudo K et al., Indirect MRI of 17O-labeled water using steady-state sequences: Signal simulation and preclinical experiment. J Magn Reson Imaging 2018; 47(5): 1373–1379.
[5] Moser JM et al., The wobbler mouse, an ALS animal model. Mol Genet Genomics. 2013; 288(5–6): 207–229.
[6] Kudo K et al., Accuracy and Reliability Assessment of CT and MR Perfusion Analysis Software Using a Digital Phantom. Radiology 2013; 267(1): 201–211.
[7] Cohen-Adad J et al., 7-T MRI of the spinal cord can detect lateral corticospinal tract abnormality in amyotrophic lateral sclerosis. Muscle Nerve 2013; 47(5):760–762.
Figures
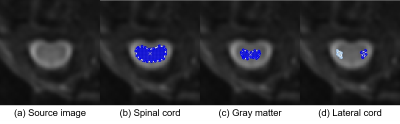
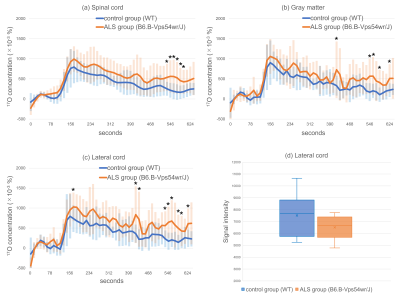
Dynamic curves of the 17O concentrations in the (a) spinal cord, (b) gray matter, (c) lateral cord. The baseline signal intensity of the lateral cord (d) is also shown. The orange and blue lines show the amyotrophic lateral sclerosis model mice (B6.B-Vps54wr/J) and control mice (wild-type), respectively. The error bars represent the standard deviation. The natural abundance of 17O, which is baseline, is 37 × 10-3 (%).
*P < 0.05