3781
Gray Matter Atrophy and Neurological Deficits in Patients with Carbon Monoxide Poisoning1The first hospital of lanzhou university, Lanzhou, China, 2The first hospital of Lanzhou university, Lanzhou, China, 3MR scientific marketing, Siemens Healthineers, Xi an, China
Synopsis
White matter damage in patients with carbon monoxide (CO) poisoning have been extensively reported in previous studies, but neurological deficits-associated gray matter volume (GMV) changes are not well explored. Forty-one CO poisoning patients and 36 healthy controls were enrolled in this study. All subjects underwent 3D T1WI and cognition and motor function assessment. GMV change patterns of patients were explored using Voxel-based morphometry processing. This study would provide a simple neuroimaging biomarker to identify the brain anatomical signatures underlying neurological deficits in CO poisoning, and thus facilitate the ongoing effort of knowing of the neuropathologic mechanism happened in these patients.
Introduction
Previous voxel-based morphometry (VBM) study with strict statistical criteria ( corrected P < 0.05 with family-wise error (FWE) ) showed the significant atrophies in the bilateral hemisphere cortices and basal ganglia in the CO poisoning patients with dementia [1]. In contrast, with the same criteria, another study did not found significant gray matter (GM) atrophy in those patients[2]. Subsequently, a longitudinal study detected regional GM atrophy from 1 week to 1, 3 and 9 months after CO poisoning, and the main involved regions belong to the pain matrix [3]. In addition, previous VBM studies most discussed the cognition decline-related GM change, but the motor impairment, accompanying by the most patients, usually got less attention. Based on the debates and limitation, here, we aimed to explore regional gray matter volume (GMV) changes of the CO poisoning patients with voxel-by-voxel comparisons again, and to identify the differences between the patients with delayed neurological sequelae (DNS) and those without DNS, futhermore, to investigate the association between the altered brain region and neurological deficits.Methods
Following informed consent, Forty-one CO poisoning patients (30 DNS and 11 non-DNS ) and 36 healthy controls (HCs) were enrolled in this study. Neuropsychological (NP) tests including Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Unified Parkinson’s Disease Rating Scale subsection III (UPDRS III), and Barthel Index (BI) were administered before the MR scans on the same day.All MRI scanning were performed on a 3T system (MAGNETOM Skyra; Siemens Healthcare, Erlange, Germany) with a 20-channel phased-array head coil. Whole-brain T1WI data were acquired using a 3D MPRAGE sequence with the following parameters: TR/TE/TI = 2300/2.98/900 ms, respectively; flip angle = 9 degrees, field of view = 256 × 256 mm, slice thickness =1 mm. sMRI data were postprocessed by the same neuroradiologist using the CAT12 toolbox in the Statistical Parametric Mapping 12 toolboxon the MATLAB 2016a platform. One-way analysis of covariance was performed with the covariation of age, sex, and TIV to investigate differences in regional GM volume among groups. Differences were considered significant when cluster-level FWE-corrected P < 0.05 ( uncorrected P < 0.001 with a cluster size >80 ). Partial correlation analysis adjusted for age, sex, education and TIV was performed to assess the correlation between NP scores and regional GMV. The area under the curve of regional was calculated by receiver operating characteristics.Results
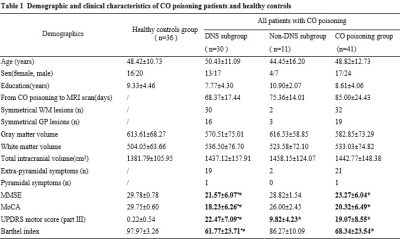
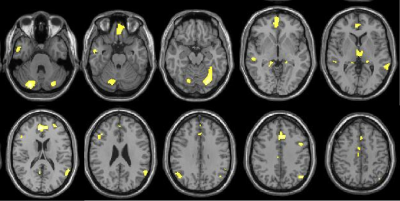
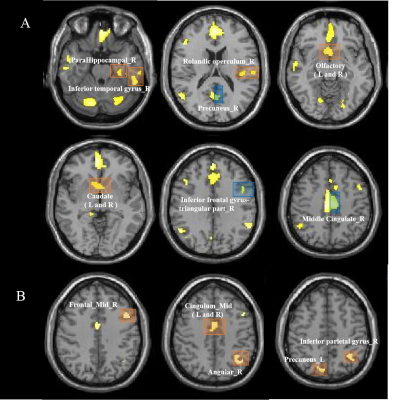
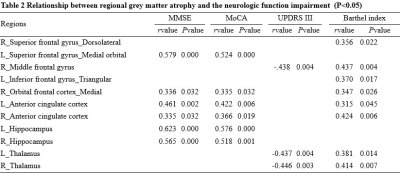
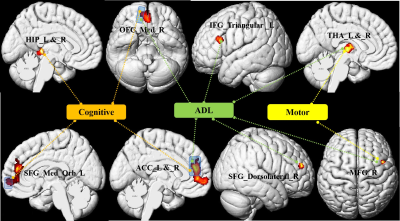
Table 1 summarizes the clinical data of the subjects. 51.2% of patients had the extra-pyramidal symptoms, including bradykinesia, rigidity, gait disturbances, and postural instability. Compared with the HCs group, GMV decreased significantly in the bilateral frontal-temporal-parietal lobe, hippocampus, thalamus, cingulate cortex and left middle cingulate cortex (MCC), and bilateral cerebellumin the CO poisoning group (Figure 1). In addition to the same atrophy regions as the CO poisoning group, the DNS subgroup showed much more atrophy clusters (Figure 2A).The decreased GMV regions in the DNS subgroup as compared with the non-DNS subgroup were showed in the Figure 2B.Table 2 and Figure 3 showed the correlation between NP scores and GMVs (P < 0.05). In the six GM atrophy regions, the bilateral MCC had the best performance to differentiate DNS patients from non-DNS patients ( P < 0.05 ).Discussion & Conclusion
In the current study, cerebellum posterior lobe atrophy was firstly detected in our patients. Meanwhile, in the frontal and parietal lobe, the significant atrophic subregions involved in the well-known default mode network. The DNS patients displayed more widespread atrophy areas than the CO poisoning group relative to the control group, which further involved in the limbic system and striatum. Bilateral MCC are main differential regions of the patients with DNS and without DNS. Furthermore, our results showed that the cognitive impairment-related GM atrophy regions belong to the limbic system. Twenty-two patients had motor paralysis, of whom twenty-one patients appeared parkinson's symptoms. Moreover, the decreased GMV in the right middle frontal gyrus (MFG) and bilateral thalamus were correlated with increased UPDRS III scores. It is well know that MFG belong to premotor area and is a part of extra-pyramidal cortical region. Otherwise, in line with previous study, thalamus as a part of network mediated CO-related parkinsonism features. Last, we found prefrontal cortex, anterior cingulate cortex, and thalamus as a network played a important role in regulating the ability of daily living.Acknowledgements
We wish to thank the patients and their caregivers for their time and commitment to this research. We are also grateful that the study was supported by research grants from the Natural Science Foundation of China (NO. 8216150042, 2021), and the technology plan project of Lanzhou city (NO. 2020-ZD-71, 2021).References
[1] Chen NC, Chang WN, Lui CC, et al. Detection of gray matter damage using brain MRI and SPECT in carbon monoxide intoxication: a comparison study with neuropsychological correlation. Clin Nucl Med 2013;38(2):e53-9.
[2] Chen HL, Chen PC, Lu CH, et al. Structural and cognitive deficits in chronic carbon monoxide intoxication: a voxel-based morphometry study. BMC Neurol 2013;13:129.
[3] Chou MC, Li JY, Lai PH. Longitudinal gray matter changes of the pain matrix in patients with carbon monoxide intoxication: A voxel-based morphometry study. Eur J Radiol 2020;126:108968.
Figures