3780
Dissecting Brain-wide Olfactory Dysfunctions in Accelerated Aging Rodent Model
Teng Ma1,2,3, Xunda Wang1,2, Linshan Xie1,2, Pit Shan Chong4, Venice Sin1,2, Peng Cao3, Pek-Lan Khong3, Lee Wei Lim4, Alex T. L. Leong1,2, and Ed. X Wu1,2,4
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 4School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 4School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Synopsis
The olfactory system plays a pivotal role in driving behavioral responses that are critical to survival. In particular, the decline in ability to detect and discriminate odors in aged humans lead to an overall decrease in quality of life. However, our present understanding of olfactory dysfunction in aging brains beyond the cellular and micro-circuit level is scarce and incomplete. In this study, we deployed optogenetic fMRI to reveal the changes of brain-wide odor-associated regions brought about by aging in an accelerated aging rat model. We found diminished activations brain-wide indicating dysfunction at the systems level across multiple long-range olfactory pathways.
Purpose
The number of people aged 65 years old or over is projected to double by 2050 indicating the ever increasing aging population worldwide1. As a core organ of the human body, the brain is inextricably affected by aging, resulting in many chronic age-associated neurodegenerative symptoms including olfaction degradation and memory impairment2-4. Olfaction, one of our primary senses, plays a pivotal role to elicit and drive behavioral responses that are critical to survival5,6. There is a significant decline in olfactory activities as humans aged5 leading to a decrease in the quality of life and diminished appetite with accompanying impact on nutritional status7.Previous studies on aging-related olfactory deficits covered various aspects, ranging from changes at the molecular level (i.e., odor receptors in the nose, and protein expression6,8), cellular degeneration of olfactory neurons (i.e., synapse loss and reduction of neurogenesis9) to behavioral impairment (i.e., decrease in odor identification and discrimination, and olfactory memory decline10). However, there is a notable gap in the examination of such olfactory dysfunction caused by aging at the systems level due to the lack of suitable neurotechnologies. To bridge this gap, we deployed optogenetic functional MRI to characterize the changes in the long-range olfactory pathways in an accelerated aging rat model via stimulation of the olfactory bulb (OB) excitatory projection neurons. Note that OB is the nexus for all olfactory processes in the central nervous system11,12.
Methods
Accelerated aging rat model and optogenetic stimulation: Adult rats (male, SD strain, 10 weeks old, n=14) were treated with D-galactose (100mg/kg) for 8 weeks13,14. At week five, 3μl of AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to OB (7.5mm anterior to Bregma, +1.7mm medial-lateral right hemisphere, -2.2mm from the surface of dura). Four weeks after viral injection, an opaque optical fiber cannula (d=450μm) was implanted at the injection site (Figure 1A). Blue (473nm) light was presented to animals expressing ChR2 at 1Hz (10% duty cycle, 40mW/mm2), 5, 10, 20 and 40Hz (30% duty cycle, 40mW/mm2) in a block-design paradigm (Figure 1B).fMRI acquisition and analysis: fMRI data was acquired on 7T Bruker scanner using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms, and 20 contiguous slices with 1mm thickness). Data were preprocessed before standard GLM analysis was applied to identify significant BOLD responses (p<0.001; FDR corrected).
Results
Brain-wide fMRI mapping of downstream olfactory signal propagation from OB in aged animalsWe observed that optogenetic stimulation of ipsilateral OB excitatory neurons at all frequencies evoked activations in primary olfactory regions (anterior olfactory nucleus AON, piriform cortex Pir, olfactory tubercle Tu, entorhinal cortex Ent, amygdala Amg), orbitofrontal cortex (OFC), cingulate cortex (Cg), insular cortex (Ins) and striatal regions (nucleus accumbens NAc, caudate putamen CPu) in aged rats (Figure 2B, C). Note that only 1Hz was able to evoke bilateral activations in these aforementioned brain regions with further activations in sensory cortices, including somatosensory (S1), visual (VC) and auditory (AC) cortices. BOLD responses in the primary olfactory regions generally increased with stimulation frequency. On the contrary, the BOLD responses in higher-order brain regions (i.e., OFC, Cg, NAc, and CPu) decreased with increasing stimulation frequencies (Figure 2B, C).
Diminished long-range neural activity propagation in aged versus normal rats
Comparing the activations evoked by optogenetic stimulation of ipsilateral OB in aged rats to normal adult rats (12 weeks old) from our previous report15 (Figure 3B, C), it is notable that the BOLD responses of the primary olfactory and striatal regions, and OFC, Ins and S1 declined significantly at low frequency (1Hz) in aged rats (Figure 4). All contralateral activations also decreased significantly in aged animals. Evoked activations at high frequencies (10, 20 and 40Hz) in ipsilateral primary olfactory regions and Ins were comparable. Our findings indicate general dysfunction across numerous long-range olfactory pathways and diminished interhemispheric communication for olfactory processing in aged brains.
Discussion & Conclusion
In this study, we examined the changes in the long-range olfactory pathways of an accelerated aging rat model using optogenetic fMRI. Studies in rodents have shown that the decreased ability to perceive odors were not associated with general decline in number of receptors/cells in the olfactory epithelium or a decline in neuronal populations (i.e., mitral and tufted cells, and interneurons) in the olfactory bulb9. Instead, the synaptic density in the glomeruli of the olfactory bulb appears diminished9, suggesting impaired downstream olfactory information processing from the olfactory bulb to targets in the subcortical and cortical regions. Our results indicate that such impairment is likely true with the diminished brain-wide neural activity propagation to olfactory regions and beyond, and the absence of interhemispheric activations in aged animals.In summary, our study characterized the spatiotemporal response properties of downstream neural activity propagation from OB across long-range pathways in an aged rat model. More importantly, we revealed critical insights into the age-related olfactory dysfunction at the systems level.
Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (R7003-19F, HKU17112120 and HKU17127121 to E.X.W., and HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L.), Lam Woo Foundation, Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001) and Guangdong Key Technologies for Alzheimer’s Disease Diagnosis and Treatment (2018B030336001) to E.X.W.References
- United Nations Department of Economic and Social Affairs, P.D. World Population Ageing 2020 Highlights: Living arrangements of older persons (ST/ESA/SER.A/451). (2020).
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180-186 (2016).
- Kubben, N. & Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol 18, 595-609 (2017).
- Hou, Y.J., Dan, X.L., Babbar, M., Wei, Y., Hasselbalch, S.G., Croteau, D.L. & Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nature Reviews Neurology 15, 565-581 (2019).
- Wang, J., Sun, X. & Yang, Q.X. Early Aging Effect on the Function of the Human Central Olfactory System. J Gerontol A Biol Sci Med Sci 72, 1007-1014 (2017).
- Kovacs, T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev 3, 215-232 (2004).
- Garrison, J.L. & Knight, Z.A. Linking smell to metabolism and aging. Science 358, 718-719 (2017).
- Durante, M.A., Kurtenbach, S., Sargi, Z.B., Harbour, J.W., Choi, R., Kurtenbach, S., Goss, G.M., Matsunami, H. & Goldstein, B.J. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat Neurosci 23, 323-326 (2020).
- Mobley, A.S., Rodriguez-Gil, D.J., Imamura, F. & Greer, C.A. Aging in the olfactory system. Trends Neurosci 37, 77-84 (2014).
- Moreno, M., Richard, M., Landrein, B., Sacquet, J., Didier, A. & Mandairon, N. Alteration of olfactory perceptual learning and its cellular basis in aged mice. Neurobiol Aging 35, 680-691 (2014).
- Mori, K. & Sakano, H. Olfactory Circuitry and Behavioral Decisions. Annu Rev Physiol 83, 231-256 (2021).
- Kay, L.M. Olfactory system oscillations across phyla. Current opinion in neurobiology 31, 141-147 (2015).
- Ali, T., Badshah, H., Kim, T.H. & Kim, M.O. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J Pineal Res 58, 71-85 (2015).
- Kay, L.M. Olfactory system oscillations across phyla. Curr Opin Neurobiol 31, 141-147 (2015).
- Ma, T., Leong, A.T.L., Wang, X., Wong, E.C., Sanchayyan, S., Chong, P.S., Lim, L.W., Khong, P.L. & Wu, E.X. Optogenetic fMRI interrogation of the olfactory system. Proceedings of International Society for Magnetic Resonance in Medicine Virtual Conference p2941 (2021).
Figures
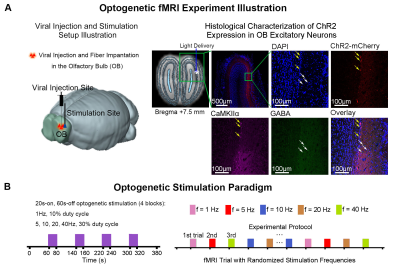
Figure
1. Histological characterization of ChR2 viral expression in the olfactory bulb
(OB) and optogenetic fMRI stimulation setup. (A) Illustration of OB optogenetic
stimulation (left), T2-weighted anatomical MRI image showing the location of
the implanted optical fiber (asterisk indicates the stimulation site; middle). and
confocal images of ChR2 expression in OB excitatory neurons (yellow arrows; right).
(B) Illustration of the optogenetic stimulation presented in a
block-design paradigm of 20s on and 60 s off (left).
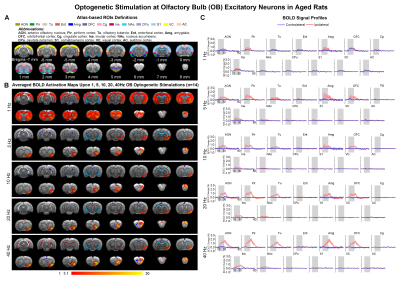
Figure
2. Brain-wide BOLD activations upon different frequency optogenetic stimulation
of OB excitatory neurons in aged rats. (A) Atlas
defined region of interests (ROIs). (B) Averaged
activation maps of OB stimulation across five different stimulation frequencies
(n=14; t>3.1 corresponding to p<0.001). (C) Corresponding BOLD
signal profiles of OB stimulation extracted from ROIs defined in A.
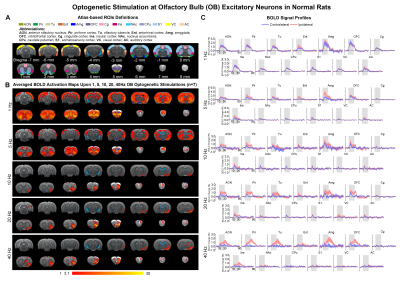
Figure
3. Brain-wide BOLD activations upon optogenetic stimulation of OB excitatory neurons
in normal adult rats15. (A) Atlas
defined region of interests (ROIs). (B) Averaged
activation maps of OB stimulation across five different stimulation frequencies
(n=7; t>3.1 corresponding to p<0.001). (C) Corresponding BOLD
signal profiles of OB stimulation extracted from ROIs defined in A.
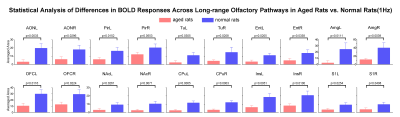
Figure
4. Impaired OB downstream long-range neural activity propagation in aged versus
normal adult rats. One-tail
unpaired t-test analysis with Bonferroni correction showed the significant
decline of activations in the primary olfactory and striatal regions, and orbitofrontal
cortex OFC, insula Ins and somatosensory cortex S1. Error bars indicate ± SEM.
DOI: https://doi.org/10.58530/2022/3780