3760
Fumarate Metabolic Signature for Detecting Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome-associated Renal Cell Carcinoma1Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 2United Imaing Healthcare, Shanghai, China
Synopsis
Non-invasive in vivo detection of abnormal fumarate accumulation is important for identify fumarate hydratase (FH) deficiency in hereditary leiomyomatosis and renal cell carcinoma (HLRCC). However, until now, no study has been reported to successfully identify fumarate accumulation in HLRCC-associated RCC. In our study, we achieved an 100% accuracy in primary HLRCC-associated RCC, as well as all of the HLRCC PDX models. For our best knowledge, it is the first time that, MRS can be successfully used to in vivo detected in FH-deficient renal lesions, which is a crucial innovation of conventional imaging diagnostic technology.
INTRODUCTION
Non-invasive in vivo detection of abnormal fumarate accumulation is important for identify fumarate hydratase (FH) deficiency in hereditary leiomyomatosis and renal cell carcinoma (HLRCC). However, until now, no study has been reported to successfully identify fumarate accumulation in HLRCC-associated RCC. The aim of this study was to investigate the feasibility of MRS in detecting abnormally elevated fumarate levels in HLRCC-associated RCC based on in vivo patient-derived tumour xenografts (PDXs) as well as patients to explore the possibility of applying MRS in HLRCC diagnosis.
METHODS
This study was retrospectively designed. MRS was performed on HLRCC-associated patient-derived tumour xenograft (PDX) models and control models as well as clinical suspicion of HLRCC-associated RCC patients(n =36) were enrolled between January 2018 and August 2021 from Renji Hospital. MRS results were interpreted as ‘detected’, ‘borderline’, ‘undetected’ or ‘technical failure’. Sensitivity, specificity and accuracy were assessed. Three independent investigators were blinded to the clinical observations and genetic test results at the time of analyses examined all spectra in a qualitative manner.RESULTS
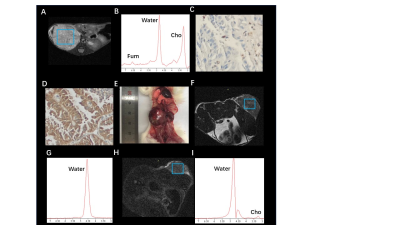
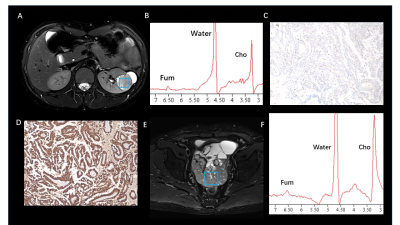
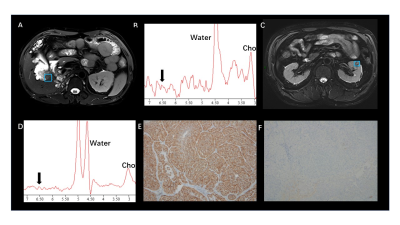
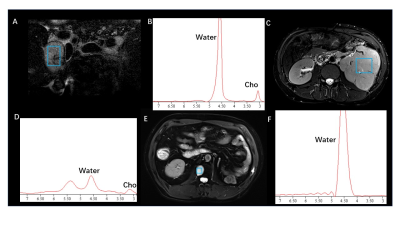
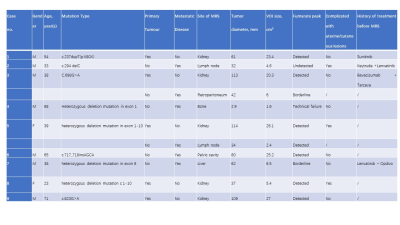
Fumarate peaks were successfully detected at 6.54 ppm in all PDX models but undetected in control models(Fig. 1). 36 patients (16 women and 20 men; mean age, 50 years [range, 18 to 71 years]) with 44 lesions were studied. Finally, a total of 9 patients with 11 lesions were diagnosed with HLRCC-related RCC or related metastatic lesions based on the FH germline and immunohistochemistry of tumour tissue showing absent FH immunostaining, the demographic and clinical characteristics are shown in Table 1. In patients, all primary renal lesions proved to be FH-deficient were presented with detected fumarate peak(Fig 2); moreover, no fumarate peak was detected in lesions without FH-deficient. Borderline fumarate peaks were detected in 6 patients with 6 lesions, including 2 primary renal tumours and 4 metastatic lesions, of whom 2 lesions with FH-deficient and 4 lesions without (Fig 3). The fumarate peak was undetected in 19 patients with 23 lesions, including 13 primary renal tumours and 10 metastatic lesions, of whom only 1 metastatic lesion was confirmed to be HLRCC associated; Technical failure occurred in 8 patients with 8 lesions, all of whom were metastatic lesions, including 1 lesion was HLRCC associated and 7 lesions with preserved FH expression((Fig 4). Evaluating primary and metastatic lesions separately, the accuracy was 100% (25/25) and 92% (23/25) in primary lesions, while 78.9% (15/19) and 78.9% (15/19) in metastases with the ‘detected’ or ‘detected + borderline’ as a positive result respectively. For the diagnostic performance, regarding the detection of the fumarate peak as a positive result and the remainder as a negative result, the sensitivity, specificity and accuracy of MRS based on per-lesion were 63.6% (7/11), 100% (33/33), and 90.9% (40/44), respectively. If borderline cases were also classified as positive results, the sensitivity, specificity and accuracy were 81.8% (9/11), 87.9% (29/33) and 86.4% (38/44), respectively.DISCUSSION
Morphologic features based on imaging modalities are the most classical method for the differential diagnosis of RCC subtypes1-4; However, the radiologic appearance of RCC lesions in HLRCC patients is highly variable based on the high degree of heterogeneity, and with large overlaps with the traditional imaging features of other subtypes of RCC5. MRS is a potential technique to reflect the abnormal accumulation of metabolites in vivo6-9. In our study, we achieved an 100% accuracy in primary HLRCC-associated RCC. Moreover, all of the HLRCC PDX models shown detected fumarate peak demonstrates the probability to apply MRS on charactering the condition and distribution of abnormal fumarate accumulation.For our best knowledge, it is the first time that, as a metabolic-function based noninvasive diagnostic tool, MRS can be successfully used to in vivo detected the abnormal accumulation of fumarate in FH-deficient renal lesions, and in this instance, MRS could provide important metabolic information beyond traditional imaging sequences. Clearly detected fumarate peak was found in 2 of 6 metastatic lesions, while 2 metastatic cases were classified as “borderline” (the result was difficult to clearly classify as positive or negative and was categorized as borderline), the result may mainly contributed to the fact that within the volume of interest in MRS scanning, the density of accumulation of fumarate in primary lesions may higher than that in metastatic lesions as well as smaller size, more irregular shape and more complex location compared with primary tumours. Our study demonstrate the feasibility to regard borderline as positive finding, with the criteria only 2 HLRCC cases were missed. Another evidence to support the approach is that only few cases with borderline were found in lesions without FH-deficient while no detected fumarate peak was found. Based on this finding, we believe that the diagnostic criteria in clinical practice can be flexible. Detected results could be used to clearly identify positive patients, while undetected result could be used to eliminate cases that HLRCC-unrelated in a robust and convenient manner, avoiding unnecessary time-consuming and invasive tests (eg., genetic test, biopsy).CONCLUSION
As a metabolic function-based diagnostic tool, MRS is a highly specific and sensitive hallmark of FH mutations.Acknowledgements
This study was supported by Shanghai Jiao Tong University medical-engineering cross fund [grant number YG2021QN27], Shanghai Jiao Tong University School of Medicine Affiliated Renji Hospital Clinical Research Innovation and Cultivation Fund [grant numbers PYII20-11], the Science and Technology Commission of Shanghai Municipality [grant number 18DZ1930104].References
[1] Egbert ND, Caoili EM, Cohan RH, et al. Differentiation of papillary renal cell carcinoma subtypes on CT and MRI. AJR American journal of roentgenology. 2013;201:347-355.
[2] Fu W, Huang G, Moloo Z, et al. Multimodality Imaging Characteristics of the Common Renal Cell Carcinoma Subtypes: An Analysis of 544 Pathologically Proven Tumors. Journal of clinical imaging science. 2016;6:50.
[3] Ching BC, Tan HS, Tan PH, et al. Differential radiologic characteristics of renal tumours on multiphasic computed tomography. Singapore medical journal. 2017;58:262-266.
[4] Yamada T, Endo M, Tsuboi M, et al. Differentiation of pathologic subtypes of papillary renal cell carcinoma on CT. AJR American journal of roentgenology. 2008;191:1559-1563.
[5] Paschall AK, Nikpanah M, Farhadi F, et al. Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome: Spectrum of imaging findings. Clinical imaging. 2020;68:14-19.
[6] Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nature medicine. 2012;18:624-629.
[7] Casey RT, McLean MA, Madhu B, et al. Translating in vivo metabolomic analysis of succinate dehydrogenase deficient tumours into clinical utility. JCO precision oncology. 2018;2:1-12.
[8] Tiwari V, Daoud EV, Hatanpaa KJ, et al. Glycine by MR spectroscopy is an imaging biomarker of glioma aggressiveness. Neuro-oncology. 2020;22(7):1018-1029.
[9] Casey RT, McLean MA, Challis BG, et al. Fumarate Metabolic Signature for the Detection of Reed Syndrome in Humans. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26:391-396.
Figures