3758
Changes of structural network in patients with early Lacunar Infarction
XUCHEN YU1, Hongwei Li1, Min He2, Weibo Chen3, Jing Ding2, and He Wang 1
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Neurology, The Affilated Zhongshan Hospital of Fudan University, Shanghai, China, 3Philips Healthcare, Shanghai, China
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Neurology, The Affilated Zhongshan Hospital of Fudan University, Shanghai, China, 3Philips Healthcare, Shanghai, China
Synopsis
Most of the lacunar infarcts are small and asymptomatic, while it could not illustrate brain function is normal. Due to the potential impact of early disseminated lacunar infarcts on brain network and function remains unclear, the objective of this study was to assess whether there were changes in brain network and functional connectivity.
Introduction
Cerebral small vessel disease (SVDs) is normally observed on neuroimaging in the elderly and is recognized as a major vascular contributor to dementia, cognitive impairment, gait disorders and mood disturbance1. MRI markers for SVDs, including white matter hyperintensities (WMHs), perivascular spaces (PVS), cerebral microbleeds (CMBs), lacunar infarction (LI) and brain atrophy2. However, clinical symptoms are often highly inconsistent in nature and severity among patients with similar degrees of SVD on brain imaging. Lacunar infarcts (LI) account for 20-25% of all ischemic strokes, in the general case, cognitive performance is usually normal, 55% of cases meet criteria for mild cognitive impairment (MCI) of vascular type3,4,5. Therefore, it is important to identify the early stages of LI. We analyze effects of LI on network and functional connectivity in the brain, and we discuss how network disruption by LI can lead to clinical deficits. The large variability in clinical symptoms among patients with LI can probably be understood by taking into account the heterogeneity of LI lesions and the effects of LI beyond the focal lesions.Methods
The study population consisted of 63 consecutive patients with single and disseminated LI admitted to the Department of Neurology of the Zhongshan Hospital, in Shanghai, China. Patients with stroke history or intracerebral hemorrhage were excluded from the study as were those with severe cardiovascular disease, renal insufficiency, liver dysfunction, neoplastic or chronic disease, major depression and other psychiatric comorbidity (DSM-IV-R). MRI studies were performed using a Philips 3.0 Tesla Ingenia system and the protocol included T1W_3D_TFE, 3D_T2, BOLD and Diffusion tensor imaging (DTI). The images were acquired as follows: TR=986ms, TE=30ms, Matrix=128x128, slices=78, slice thickness=2.5 mm. A battery of neuropsychological tests was administered to all patients within 1 month after MRI scanning. There included the. Preprocessing of DTI data included three steps. First, remove the effects of eddy current distortion and motion artefacts by effectively aligning the diffusion-weighted image with the reference b0 image. The transformation is then applied to reorient the b matrix. Finally, calculated the diffusion tensor, diagonalized to obtain the three eigenvalues (λ1, λ2, λ3) and their corresponding eigenvectors, and finally the FA image is calculated. For Network Node Definition the brain was wrapped into 90 regions of interest using the automatic anatomical labelling template AAL (90) to define the network nodes. The regions of interest are then segmented. Additionally, the deterministic fiber tracking method was employed to map white matter connections between brain regions. In the current research, we employed the graph theory approach to analyze the rich club organization of the brain binary networks in all subjects at the range of the nodal degree (k) cutoff, including rich club coefficient (φ(k)), clustering coefficient (C), shortest path length (L), global efficiency (Egob), and edge (E). Software (SPSS version 22.0; SPSS, USA) was employed for the correlation between volumes of 90 brain regions and network measures. A significant difference was present if the p-value was less than 0.01.Results
The functional connectivity density (FCD) was significantly higher in patients with mid-cognitive impairment than in those with subjective cognitive decline (SCD) (Fig. 1). However, in regards to the white matter fibre tracts, there was no significant variation of fibre density between two groups. Similarly, no significant differences in network measures were observed. The volume of Middle Frontal Gyrus (MFG) and Olfactory were significantly correlated with global efficiency (Fig. 2), while hippocampus have no significant association with it. In addition, the volume of left precentral gyrus, left superior gyrus and left Inferior frontal gyrus were found to be negatively correlated with their small world clustering coefficient (Fig. 3).Discussion and Conclusion
We found that both two groups (SCD and MCI) exhibited an enhancement of FCD, but MCI was higher than SCD. Based on previous study, brain reserve and cognitive reserve, which can provide protection against of brain pathology, and plays a compensatory role for clinical deterioration in the presence of pathology1. For the correlation analysis between the structural network and brain volume, global efficiency and clustering coefficient were significantly associated with the volume. Network efficiency as a form of brain reserve, more efficient networks might protect against clinical deterioration in the presence of brain pathology. Here, an increase in global efficiency in the structural network might indicate self-regulation of the brain. Rhinal cortex as the region leading the recognition of familiarity in memory is significantly associated with global efficiency. Patients with little or disseminated LI might perform poorly in Delayed non-match Sample (DNMS) rather than a decline in memory. We suggest that mild LI patients might manifest as emotional, long-term memory and non-working memory impairment. For further study, we will follow these patients for a long time to observe their changes in these functions to determine the nodes of the changes in patients with early cavity infarction.Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 81971583), National Key R&D Program of China (No. 2018YFC1312900), Shanghai Municipal Science and Technology Major Project (No. 20ZR1406400) and ZJLab.References
1. Annemieke T, Esther MC et al., Cerebral small vessel disease: from a focal to a global perspective. Nature Reviews 2018; 14:387-3972.Andreas P, Konstantinos P et al., Left Ventricular Hypertrophy and Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. J stroke 2020. 22(2):206-2243. Lorena B, Adria A et al., Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: a comparative study. BMC Neurol 2013. 13:2034. Arboix A, Martí-Vilalta JL et al., Lacunar stroke. Expert Rev Neurother 2009. 9:179–196. 5. Fisher CM. Lacunar infarcts A review. Cerebrovasc Dis 1991. 1:311-320.Figures
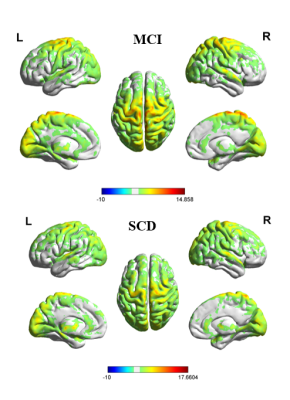
Functional connection density of MCI group and SCD group
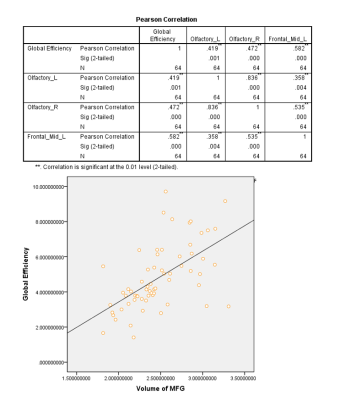
Global efficiency is significantly positively correlated with the volume of the Olfactory_L, Olfactory_R and Frontal_Mid_L
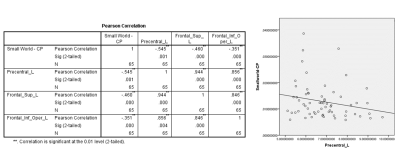
Clustering coefficient is significantly negatively correlated with the volume of the Precentral_L, Frontal_Sup_L and Frontal_Inf_Oper_L
DOI: https://doi.org/10.58530/2022/3758