3757
Implementation of a Multiparametric MRI Model of Glioma Infiltration for Dose Painting Radiotherapy1ACRF Image X Institute, Sydney School of Health Science, The University of Sydney, Eveleigh, Australia, 2Medical Physics, Ingham Institute of Applied Medical Research, Liverpool, Australia, 3Liverpool and Macarthur Cancer Therapy Centres, Liverpool, Australia, 4South West Sydney Clinical School, University of New South Wales, Sydney, Australia, 5Brain Tumor Center Amsterdam, Amsterdam University Medical Centre, Amsterdam, Netherlands, 6Department of Neurosurgery, Amsterdam University Medical Centre, Amsterdam, Netherlands
Synopsis
Multiparametric MRI (mpMRI) promises to guide dose painting (DP) radiotherapy to improve local control rates in glioblastoma (GBM) patients. In this study we develop a workflow for DP based on a clinically validated mpMRI model of infiltrative tumour in GBM patients. We demonstrate the repeatability of DP prescriptions, the quality of the resulting DP plans, and that DP can improve modelled local control rates over standard radiotherapy in GBM patients. Our findings provide evidence of technical and clinical validation steps, which are key for the clinical translation of mpMRI-based DP in GBM.
INTRODUCTION
Glioblastoma multiforme (GBM) is the most aggressive type of primary brain cancer, with most tumours recurring locally within months of first-line treatment with debulking surgery and adjuvant chemo-radiation.1,2 The challenges to improve local control (LC) are linked to the invasive and heterogeneous nature of GBM, which makes it extremely difficult to distinguish infiltrating tumour from surrounding healthy tissue, identify treatment resistant tumour tissue, and assess tumour response to treatments on imaging studies. Contrast enhanced MRI is a cornerstone imaging technique in the clinical management of GBM patients, and is used for diagnosis, treatment planning and response assessment.3,4 Radiotherapy currently utilises anatomical MRI data to delineate the gross tumour volume, which is expanded with isotropic margins to delineate the radiotherapy target. This target volume is treated with a uniform dose of 60 Gy, which is limited to prevent normal tissue complications.5 This approach fails to achieve good LC rates as the heterogeneous physiology present within the same tumour causes variations in tissue radiosensitivity, leading to treatment failure of conventional uniform radiation dose regimens.6Dose painting (DP) radiotherapy has emerged as an approach to improve GBM LC rates by using physiological information to selectively escalate the dose to regions with high likelihood of tumour infiltration, with minimal increase in toxicity to the surrounding healthy tissue.6,7 Multiparametric MRI (mpMRI) methods provide a means to characterise different aspects of the tissue’s physiology and can be used to guide DP approaches.8,9 Over the last decade, a number of mpMRI models predicting the probability of tumour infiltration beyond the tumour margins visible on anatomical MRI have been published.10–15 Key to the implementation of mpMRI models for clinical use is demonstrating that the derived DP prescriptions are repeatable and that the resulting DP plans would lead to clinical benefits compared to current standard of care radiotherapy.16 In this study, we evaluate the practical application of a mpMRI model of glioma infiltration for radiotherapy in GBM patients by developing DP prescriptions, testing their repeatability and estimating expected benefits in LC rates for the DP plans over conventional standard radiotherapy plans.
METHODS
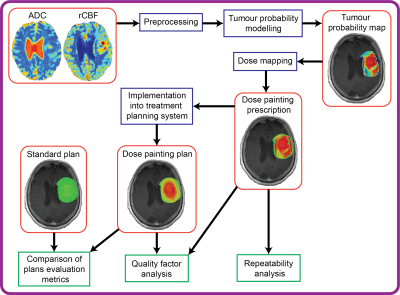
Publicly available post-operative mpMRI data from 11 GBM patients were used.17 Apparent diffusion coefficient (ADC) and relative cerebral blood flow (rCBF) maps obtained from diffusion and perfusion-weighted MRI sequences, respectively, were linearly combined in a clinically validated mpMRI model of glioma infiltration to generate maps of tumour probability (TP).11 DP prescriptions ranging from 60 to 80 Gy were derived from the TP maps by means of a linear dose mapping function.18 Repeatability of DP prescriptions within the radiotherapy margins was evaluated by means of intraclass correlation coefficient (ICC) using test-retest scans available for each patient. A workflow was developed to import the DP prescription into a treatment planning system and retrospectively develop a DP plan (Figure 1). This workflow was tested for the first patient. The quality of the DP plan was measured with a quality factor (QF), defined as:$$QF = 100 - \frac{1}{n}\sum_i^n\mid\frac{Dose{\tiny plan,i} - Dose{\tiny prescribed,i} }{Dose{\tiny prescribed,i}}\mid\times100$$
A standard radiotherapy plan delivering a uniform dose of 60 Gy within the radiotherapy target was also developed and dose metrics and tumour control probability (TCP)19 in the radiotherapy target volume and in critical brain structures were compared between the DP plan and the standard plan.
RESULTS
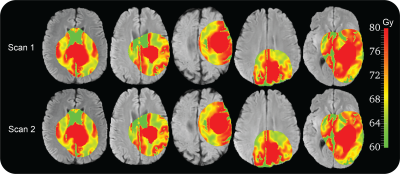
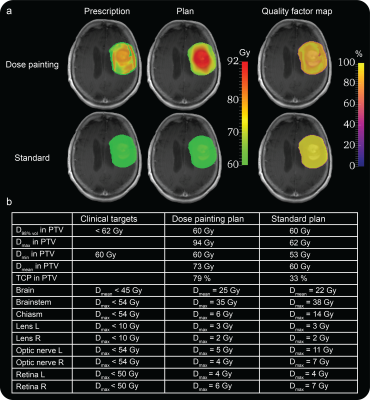
DP prescriptions derived from the test-retest scans of 11 patients showed high similarity and good levels of repeatability, with median ICC of 0.89 (range 0.79-0.95), Figure 2. The DP plan generated from the DP prescription for the first patient of the study showed a QF of 89%, indicating a good agreement between planned and prescribed dose (Figure 3a). The comparison between the standard and DP plans (Figure 3b) demonstrated that the DP plan selectively increases the mean dose to the target volume by 13 Gy and improves TCP over the standard plan by 46%, without affecting treatment toxicity according to standard clinical metrics.DISCUSSION
Results from the ICC analysis showed good repeatability of the DP prescriptions derived from the mpMRI model of glioma infiltration and QF analysis revealed good agreement between prescribed and planned dose distributions, demonstrating that DP prescriptions can be used to generate reliable DP plans. The selective dose escalation and the consequent improvement in TCP in the radiotherapy target, together with the lack of increase in dose delivered to critical brain structures achieved with the DP plan over the standard plan suggests that DP based on the mpMRI model proposed in this study holds the potential to improve LC rates in GBM patients. While promising, these preliminary results warrant validation from the other patients in the study.CONCLUSION
This study demonstrates critical repeatability, feasibility and plan quality results for the technical and clinical validation process of a mpMRI model of glioma infiltration for DP radiotherapy. The results pave the way for the clinical translation of a DP approach to improve LC rates in GBM patients. Future research should focus on validating whether this approach will effectively enable targeting of regions of local relapse with higher doses of radiation compared to standard radiotherapy in a retrospective study prior to evaluating the survival benefits of this approach in a phase II clinical trial.Acknowledgements
No acknowledgement found.References
1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17:iv1-iv62.
2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005;352(10):987-996.
3. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113.
4. Pope WB, Brandal G. Conventional and advanced magnetic resonance imaging in patients with high-grade glioma. Q J Nucl Med Mol Imaging. 2018;62(3):239-253.
5. Niyazi M, Brada M, Chalmers AJ, et al. ESTRO-ACROP guideline “target delineation of glioblastomas.” Radiother Oncol. 2016;118(1):35-42.
6. Thorwarth D. Biologically adapted radiation therapy. Z Med Phys. 2018;28(3):177-183.
7. Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): Biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47(3):551-560.
8. Castellano A, Bailo M, Cicone F, et al. Advanced Imaging Techniques for Radiotherapy Planning of Gliomas. Cancers (Basel). 2021;13(5):1063.
9. O’Connor JPB, Rose CJ, Waterton JC, Carano RAD, Parker GJM, Jackson A. Imaging intratumor heterogeneity: Role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21(2):249-257.
10. Fathi Kazerooni A, Nabil M, Zeinali Zadeh M, et al. Characterization of active and infiltrative tumorous subregions from normal tissue in brain gliomas using multiparametric MRI. J Magn Reson Imaging. 2018;48(4):938-950.
11. Verburg N, Koopman T, Yaqub MM, et al. Improved detection of diffuse glioma infiltration with imaging combinations: a diagnostic accuracy study. Neuro Oncol. 2020;22(3):412-422.
12. Lê M, Delingette H, Kalpathy-Cramer J, et al. MRI Based Bayesian Personalization of a Tumor Growth Model. IEEE Trans Med Imaging. 2016;35(10):2329-2339.
13. Durst CR, Raghavan P, Shaffrey ME, et al. Multimodal MR imaging model to predict tumor infiltration in patients with gliomas. Neuroradiology. 2014;56(2):107-115.
14. Hu LS, Ning S, Eschbacher JM, et al. Multi-Parametric MRI and Texture Analysis to Visualize Spatial Histologic Heterogeneity and Tumor Extent in Glioblastoma. Chen K, ed. PLoS One. 2015;10(11):e0141506.
15. Gaw N, Hawkins-Daarud A, Hu LS, et al. Integration of machine learning and mechanistic models accurately predicts variation in cell density of glioblastoma using multiparametric MRI. Sci Rep. 2019;9(1):1-9.
16. O’Connor JPB, Aboagye EO, Adams JE, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14(3):169-186.
17. Clark K, Vendt B, Smith K, et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J Digit Imaging. 2013;26(6):1045-1057.
18. Bowen SR, Flynn RT, Bentzen SM, Jeraj R. On the sensitivity of IMRT dose optimization to the mathematical form of a biological imaging-based prescription function. Phys Med Biol. 2009;54(6):1483-1501.
19. Thorwarth D, Notohamiprodjo M, Zips D, Müller A-C. Personalized precision radiotherapy by integration of multi-parametric functional and biological imaging in prostate cancer: A feasibility study. Z Med Phys. 2017;27(1):21-30.
Figures