3755
Functional Human Brain Connectivity During Labor and its Alteration under Transcutaneous Electrical Nerve Stimulation
Chih-Chien Tsai1,2, An-Shine Chao3, Ngoc-Thanh Hoang4, and Jiun-Jie Wang1,2,5,6
1Department of Medical imaging and Radiological Science, Chang-Gung University, Taoyuan City, Taiwan, 2Healthy Aging Research Center, Chang Gung University, Taoyuan City, Taiwan, 3Department of Obstetrics and Gynecology, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 4Department of Radiology, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam, 5Department of Diagnostic Radiology, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 6Medical Imaging Research Center, Institute for Radiological Research, Chang Gung University/Chang Gung Memorial Hospital, Taoyuan City, Taiwan
1Department of Medical imaging and Radiological Science, Chang-Gung University, Taoyuan City, Taiwan, 2Healthy Aging Research Center, Chang Gung University, Taoyuan City, Taiwan, 3Department of Obstetrics and Gynecology, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 4Department of Radiology, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam, 5Department of Diagnostic Radiology, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 6Medical Imaging Research Center, Institute for Radiological Research, Chang Gung University/Chang Gung Memorial Hospital, Taoyuan City, Taiwan
Synopsis
Pain management during labor is an essential part of general obstetric care. Use nonpharmacological methods such as transcutaneous electrical nerve stimulation (TENS) for pain relief is necessary because the risk from anesthetics and analgesics to the mother and fetus should always be considered. Therefore, we investigate the neural network alteration of the brain in pain relief by TENS during labor. Our findings indicated the TENS during labor might change functional connectivity of the pain-related network to reach the effect of pain relief, which improve our understanding of the neural representations of nociceptive and visceral pain-related networks.
INTRODUCTION
Labor pain includes a chain of physiological processes, such as uterine contractions, cervical dilation, fetal descent, perineal stretching to descent, and the birth of the baby [1]. Although pain accompanies during labor, the perception of each individual’s response to the labor pain is highly variable, leading to noticeable changes in the brain pain network. The origin of brain pain network changes during labor is still an unanswered question. Labor pain relief becomes a priority campaign to reduce pain perception, which also eliminates the negative impact on the cognitive system and brain connectivity. Transcutaneous electrical nerve stimulation (TENS) is a non-invasive nonpharmacological intervention for pain relief during labor [2; 3; 4]. Azar et al. concluded that TENS could reduce labor pain without any unexpected side effects [5]. However, evidence for how TENS affects brain networks should be further determined by imaging-related studies. We hypothesized that TENS would significantly impact neural activities through its pain-relieving function. The exploring characteristics of both local and global pain networks in the brain were performed in the current study. Additionally, analyzing the network alternations correspond to the sensation of labor pain with and without applying TENS. The information will display on the brain activation maps and the functional neural network model.METHODS
Twenty-two pregnant women at the active stage of laboring were recruited. Fifteen women (aged 29.0 ± 5.4 years old) were used TENS as the anesthesia approach and seven subjects (aged 29.6 ± 2.3 years old) were normal delivery without TENS. A portable battery-powered TENS unit (HANS model, LH202H, Singapore) was placed at bilateral Hegu (Li 4) and Sanyinjiao (Sp 6) [4] of the skin for the participant. TENS setting was as follows: output, 15 milliamperes; frequency, 100 Hz; burst frequency, 2 Hz; pulse duration, 0.25 ms, total time, 30 min. A TENS session for 30 min was applied when the parturient needed pain relief during the first stage of labor. Images were acquired from a 1.5 T MR scanner (Intera, Philips, Best, Holland). Functional MRI was acquired using a single-shot gradient-recalled echo-planar imaging (EPI) sequence with the following parameters: 28 slices; TR, 3000 ms; TE, 40 ms; voxel size, 3× 3×5 mm3; matrix size, 64×64; Scan time: 360 seconds. Five sessions in maximum were conducted according to the status of the participant. Function MRI data with an estimation of functional connectivity was analyzed by using DPABI (https://www.rfmri.org/dpabi). Images were parcellated into 246 regions of interest to explore the functional connectivity according to the BN246 atlas [6]. Each subject calculated the functional connectivity between ROIs, and Fisher's z-transformation was performed to normalize the correlation coefficients. Generalized estimating equations (GEE) were performed to reveal significant functional connectivity between two different ROIs with p <0.05 with the Bonferroni correction method (0.05/246).RESULTS
Figure 1 represents the connections with significant differences between the control and TENS groups during labor. Functional connectivity was with a significant increase pattern in TENS than in the control group. The involved connections were between (1) left middle frontal gyrus and right nuclear accumbens; (2) left middle frontal gyrus and left precuneus; (3) right insula and right inferior frontal gyrus; (4) left postcentral gyrus and right inferior frontal gyrus; (5) left superior parietal lobule and right orbital gyrus; (6) left, right medial orbital gyrus and right superior temporal gyrus. Figure 2 represented the connections with significant differences between TENS groups with high and low pain scores. The most significant connection nodes between high and low pain scores involved the prefrontal cortex and orbital gyrus. Significant correlations were found between the reduction in NRS score and the functional connectivity. The involved connections were located between the right middle frontal gyrus and left superior temporal gyrus (Spearman’s rho = 0.835, p=0.00011), the right middle frontal gyrus and right cingulate gyrus (Spearman’s rho = 0.829, p=0.00013), the left postcentral gyrus and left thalamus (Spearman’s rho = 0.829, p=0.00014), right insula to left precuneus (Spearman’s rho = 0.829, p=0.00013), right occipital gyrus (Spearman’s rho = 0.831, p=0.00012), and left occipital gyrus (Spearman’s rho = 0.833, p=0.00012), and right inferior parietal lobule to left occipital gyrus (Spearman’s rho = 0.833, p=0.00012) (Figure 3).DISCUSSION
During normal delivery with TENS, the cortical regions with functional connectivity change are located in the middle frontal gyrus, insula, postcentral (primary somatosensory cortex; S1), superior parietal lobule (S2). The result is consistent with the cortical regions involved in visceral and nociceptive pain [7]. The orbital gyrus, which was related to the processing of pain stimuli [8; 9], altered when connecting with the superior temporal gyrus. The anterior cingulate gyrus and inferior frontal gyrus were represented in the functional connectivity of participants with a low pain score group after TENS treatment, which all involved in the pain processing network [10; 11]. The cortical region related to sensory, affective, and modulatory activations about pain might differ in conditions of functional connectivity with and without TENS treatment.CONCLUSION
We observed significant alternations in functional connectivity by TENS treatment from resting-state fMRI, which might provide a convincing motive for applying for nonpharmacological relief and developing alternative pain-management approaches.Acknowledgements
The imaging facility was supported by the Molecular Imaging Center of Chang Gung Memorial Hospital, Linkou. The authors are grateful to the Neuroscience Research Center of Chang Gung Memorial Hospital, Linkou.References
[1] P. Steer, Physiology, Mechanisms and Management of Normal Labour, Obstetrics and Gynaecology: Clinical and Basic Science Aspects, World Scientific, 2002, pp. 153-174. [2] M.I. Johnson, and G. Jones, Transcutaneous electrical nerve stimulation: current status of evidence, Future Medicine, 2017. [3] A. Coutaux, Non-pharmacological treatments for pain relief: TENS and acupuncture. Joint Bone Spine 2017; 84 657-661. [4] A.-S. Chao, A. Chao, T.-H. Wang, Y.-C. Chang, H.-H. Peng, S.-D. Chang, A. Chao, C.-J. Chang, C.-H. Lai, and A.M. Wong, Pain relief by applying transcutaneous electrical nerve stimulation (TENS) on acupuncture points during the first stage of labor: a randomized double-blind placebo-controlled trial. Pain 2007; 127 214-220. [5] A. Aghamohammadi, Using of Trascutaneous Electrical Nerve Stimulation in Acupuncture Points for Reducing Labor Pain. Zahedan Journal of Research in Medical Sciences 2013; 15. [6] L. Fan, H. Li, J. Zhuo, Y. Zhang, J. Wang, L. Chen, Z. Yang, C. Chu, S. Xie, and A.R. Laird, The human brainnetome atlas: a new brain atlas based on connectional architecture. Cerebral cortex 2016; 26 3508-3526. [7] P.J. Matthews, and Q. Aziz, Functional abdominal pain. Postgrad Med J 2005; 81 448-55. [8] Y.F. Xie, F.Q. Huo, and J.S. Tang, Cerebral cortex modulation of pain. Acta Pharmacol Sin 2009; 30 31-41. [9] M. Kano, T. Hamaguchi, M. Itoh, K. Yanai, and S. Fukudo, Correlation between alexithymia and hypersensitivity to visceral stimulation in human. Pain 2007; 132 252-263. [10] D.L. Morton, J.S. Sandhu, and A.K. Jones, Brain imaging of pain: state of the art. J Pain Res 2016; 9 613-24. [11] W.Y. Ong, C.S. Stohler, and D.R. Herr, Role of the Prefrontal Cortex in Pain Processing. Mol Neurobiol 2019; 56 1137-1166.Figures
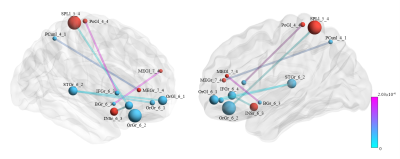
Figure
1. The links with significant difference between transcutaneous electrical nerve
stimulation (TENS) and control groups. Six connections increased connectivity in the TENS group. The involved connections included the
connection between the left middle frontal gyrus and right nuclear accumbens;
left middle frontal gyrus and left precuneus; right insula and right inferior
frontal gyrus; left postcentral gyrus and right inferior frontal gyrus; left
superior parietal lobule and right orbital gyrus; left, right medial orbital
gyrus and right superior temporal gyrus.
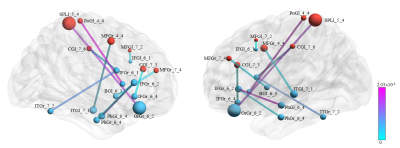
Figure
2. The links with the significant difference between transcutaneous electrical nerve
stimulation (TENS) groups with high and low pain scores. Eight connections were
found with increased connectivity in the TENS group with low scores. The most
significant connection nodes in the TENS group with high and low pain scores
involved the prefrontal cortex (anterior part of superior, middle, and inferior
frontal gyrus) orbital gyrus.
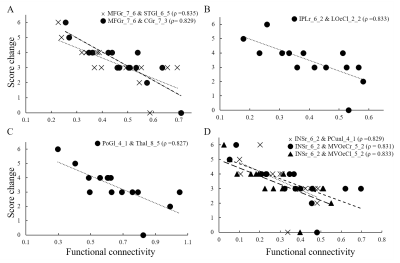
Figure
3. The correlation with reduction of pain score and functional connectivity. A
significant correlation was noted between the functional connectivity and
changes in total pain score in the transcutaneous electrical nerve stimulation
group. The involved connections were (A). middle frontal gyrus related; (B). inferior
parietal gyrus related; (C). postcentral gyrus related and; (D). insula
related.
DOI: https://doi.org/10.58530/2022/3755