3753
Alterations of the Intranetwork and Internetwork Connectivity of Default Mode Network and Olfactory Network in Patients with COVID-191Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Alzheimer's Disease Research Network, The University of Hong Kong, Hong Kong, Hong Kong, 3Department of Microbiology, The University of Hong Kong, Hong Kong, Hong Kong, 4Department of Ear, Nose and Throat Surgery, Pamela Youde Nethersole Eastern Hospital, Hong Kong, Hong Kong, 5State Key Laboratory of Emerging Infectious Diseases, The University of Hong Kong, Hong Kong, Hong Kong, 6Carol Yu Centre for Infection, The University of Hong Kong, Hong Kong, Hong Kong, 7The Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The University of Hong Kong, Hong Kong, Hong Kong, 8State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
To identify the functional abnormalities of olfactory network (ON) and default mode network (DMN) in COVID-19 patients, resting state fMRI (rs-fMRI) was applied in this study. Seed-based and ROI × ROI analysis were used to calculate the inter- and intra-network connectivity of DMN and ON. In the results, COVID-19 patients showed higher intranetwork connectivity in DMN and internetwork connectivity between ON and DMN. We postulated that these greater activities compensate for the deficits of olfactory processing and general well-being. In addition, our study suggests rs-fMRI to be a useful biomarker for the evaluation of COVID patients.
Introduction
Olfactory dysfunction (OD) is a common neurosensory defect in patients suffering from post-COVID-19 syndrome. Several studies found the Default Mode Network (DMN) modulated olfactory processing, suggesting that odor processing could draw cognitive, attentional and memory resources 1 2. A JAMA study illustrated SARS-CoV-2 may negatively affect memory even 8 months after having a mild case of the disease 3. To achieve a better understanding of the neuronal mechanism behind the observation in COVID-19 patients, we applied resting-state fMRI (rs-fMRI) to evaluate the changes of the functional intra- and inter-network connectivity of DMN and Olfactory Network (ON).Methods
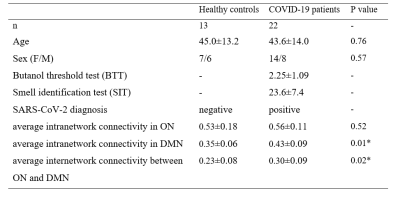
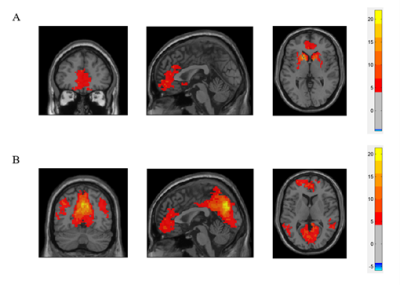
Thirteen healthy adults and twenty-two COVID–19 patients who had history of reverse transcription–polymerase chain reaction (RT–PCR) confirmed SARS‑CoV‑2 infection and presented with persistent (≥3 months) COVID–19-related OD were recruited in this study. All subjects underwent MRI examination using a 3T MR scanner (Philips, Achieva) with a 48-channel head coil. Structural images were acquired using fast and high-resolution three-dimensional (3D) sequence (BRAVO 3D sagittal, TR=900 ms, TI=900 ms, Flip angle=8o, voxel size=1×1×1 mm3, FOV=256×256 mm2). Rs-fMRI were collected by using a gradient-echo echo-planar sequence (TE=30 ms, TR=2000 ms, flip angle=80o, voxel size=3×3×4 mm3) sensitive to blood-oxygen-level-dependent (BOLD) contrast. During rs–fMRI, trial participants were instructed to look at the cross presented within the MR scanner and to not think of anything.Pre-processing of rs–fMRI data were performed using the Data Processing and Analysis of Brain Imaging (DPABI) toolbox based on the SPM12 software. We defined the seed regions for functional connectivity (FC) analyses with a sphere of 10 mm radius. The centres of the seed regions were located at the left caudate nuclei (seed region of olfactory network from a former study4, MNI coordinate (-14, 14, 2)) and left precuneus (peak value of DMN from independent component analysis, MNI coordinate (0, -72, 9)). Subsequently, we calculated the correlations between ROI series and the whole brain for each individual trial participant in a voxel–wise manner. To normalise the distribution of correlation coefficient (Pearson correlation, r), the values were transferred to standard z scores based on Fisher transformation. Then one sample t test (FDR correction, p<0.001, voxel size>5400mm3) was applied in the connectivity maps of all trial participants. After that, we got the template of DMN and ON of this cohort (Fig. 1). Based on the Automated Anatomical Labelling (AAL) anatomic parcellation, we obtained 26 ROIs (coordinates of peak t values in ON regions, sphere=10 mm3) in ON (Fig. 2A) and 51 ROIs (coordinates of peak t values in DMN regions, sphere=10 mm3) in DMN (Fig. 2B).
The ROI-wise analysis was used to compare network connectivity between groups. Each ROI of any given network was independently compared with all the other ROIs. We generated the cross-correlation matrices by Pearson correlation test (pairwise combination of all 77 ROIs). These individual correlation matrices were subsequently converted to z scores and taken to group comparison analysis within/between DMN and ON to investigate differences between the control and patient groups.
Statistical analysis was performed using SPSS (version 27, SPSS Inc., Chicage, USA). Sex difference between the groups was tested using Pearson’s chi-squre test. Two sample t test was used to investigate group differences in demographic results and functional connectivities. The relationship between the smell measurements (the butanol threshold test (BTT) and smell identification test (SIT)) and inter-/intranetwork connectivity was calculated by Pearson correlation test.
Results
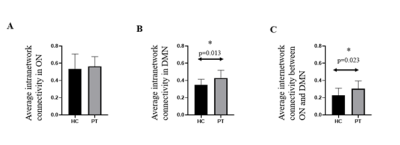
The characteristics of patients and HC are summarized in Table 1. No significant differences in age and sex was observed between the 2 groups. Significant difference could be found in the comparison of average intranetwork connectivity in DMN (p=0.013) and average internetwork connectivity between ON and DMN (p=0.023). (Table 1 and Fig. 3) In addition, the correlation between BTT score and average intranetwork connectivity in ON has a clear positive trend (r=0.385, p=0.094), which might indicate clinical performance with intranetwork connectivity.Discussion
Our findings elucidated greater activity at rest within DMN and interplay between DMN and ON in COVID patients. This increased activity at rest may be regarded as a reflection of compensatory processes, that is, an attempt to compensate for the deficits of olfactory processing and general well-being. Similar to many AD studies 5, our study suggests a useful biomarker for evaluation of COVID patients.Acknowledgements
This work was supported by the State Key Laboratory of Brain and Cognitive Sciences, the University of Hong KongReferences
1. Karunanayaka PR, Wilson DA, Tobia MJ, Martinez BE, Meadowcroft MD, Eslinger PJ, et al. Default mode network deactivation during odor-visual association. Hum Brain Mapp 2017; 38(3): 1125-39.
2. Lu J, Yang QX, Zhang H, Eslinger PJ, Zhang X, Wu S, et al. Disruptions of the olfactory and default mode networks in Alzheimer's disease. Brain Behav 2019; 9(7): e01296.
3. Soraas A, Bo R, Kalleberg KT, Stoer NC, Ellingjord-Dale M, Landro NI. Self-reported Memory Problems 8 Months After COVID-19 Infection. JAMA Netw Open 2021; 4(7): e2118717.
4. Kollndorfer K, Fischmeister FP, Kowalczyk K, Hoche E, Mueller CA, Trattnig S, et al. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. Neuroimage Clin 2015; 9: 401-10.
5. Mevel K, Chetelat G, Eustache F, Desgranges B. The default mode network in healthy aging and Alzheimer's disease. Int J Alzheimers Dis 2011; 2011: 535816.
Figures