3741
Deep slice-crossed network with local weighted loss for brain metastases detection and segmentation1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2College of Computer Science, Sichuan University, Chengdu, China
Synopsis
Brain metastases detection and segmentation on magnetic resonance images is laborious, error-prone and often irreproducible for radiologists and radiation oncologist. We present a specific deep slice-crossed network with local weighted loss to automatically detect and segment brain metastases on contrast-enhanced T1WI images. The results demonstrated the good performance, high robustness and generalizability of the model. In addition, compared with radiologists, the model showed higher sensitivity and increased efficiency in identifying and segmenting brain metastases. The results jointly suggested that the proposed model is a promising tool to assist the workflow in the clinical practice.
Purpose
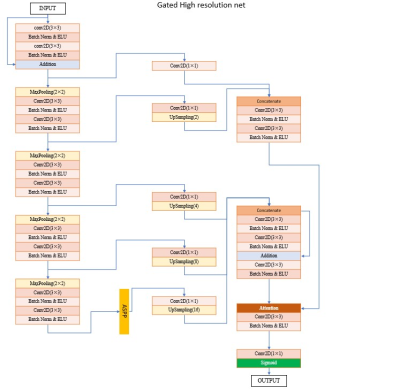
To detect and segment brain metastases (BM) on contrast-enhanced three-dimensional T1-weighted images (T1WI), we develop and evaluate a model based on deep slice-crossed network with local weighted loss.Method
In this multicenter study, contrast-enhanced 3D-T1WI images from 1482 patients with 15235 brain metastases were included. First, a dataset of 1000 patients with 11686 brain metastases from West China Hospital headquarter was used to conduct preliminary training and validation of the deep slice-crossed network model. Performance of the model was evaluated using recall, Dice Similarity Coefficient (DSC) and false-positives per patient (FP). Afterward, to test generalizability, robustness, detection efficiency and segmentation performance of the model, another five independent heterogeneous datasets were used, including 1) external data:100 BM cases from Wen jiang Branch of West China Hospital, 2) 108 BM patients with different primary tumors(36 cases with lung cancer, 36 cases with breast cancer, 36 cases with digestive tract cancer), 3) 120 data originated from different manufacturers or different strength fields(40 cases from GE 3.0 T, 40 cases from Philips 3.0 T, 40 cases from Siemens 3.0 T and 40 cases from Siemens 1.5 T, respectively), 4) 50 patients with single tiny BM, 5) isolated BM with different sizes (34 cases with a diameter of 10 mm and 30 cases with a diameter of 20 mm on the cross section, respectively).Result
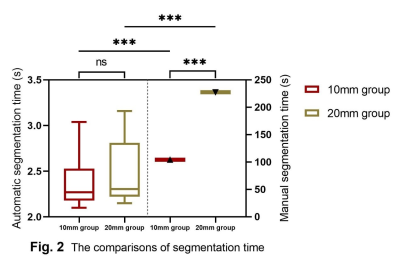
Overall, our model produced a recall of 0.88, a DSC of 0.90 and a FP per patient of 1.0, respectively. In the external test set, the network yielded a recall of 0.82, which was slightly lower than that of the internal validation set (Z = -2.92, p = 0.003). There was no significant difference in DSC between the internal and the external dataset (0.90 vs. 0.88, Z = -1.06 and p = 0.29). For data from different primary tumors, the model achieved a recall of 0.86 with a DSC of 0.85 in the lung cancer group, a recall of 0.84 with a DSC of 0.83 in the breast cancer group and a recall of 0.91 with a Dice rate of 0.90 in the digestive tract cancer group, and there were no significant differences in recall and DSC among these three groups (H = 2.04 and 0.24, p = 0.36 and 0.89, respectively). For data obtained from different manufacturers but with the same filed strengths, the network demonstrated a recall of 0.89 and a DSC of 0.90 in Siemens 3.0 T group, a recall of 0.73 and a DSC of 0.82 in GE 3.0 T group, and a recall of 0.83 and a DSC of 0.85 in Philips 3.0 T group (H = 9.60 and 7.25, p = 0.01 and 0.03, respectively). After Bonferroni correction, only the recall of Siemens 3.0 T group and GE 3.0 T group (H = -2.97, p = 0.01), and the DSC of the Philips 3.0 T group and Siemens 3.0 T group were significantly different (H = -2.64, p = 0.03). For data obtained from the same manufacturer but with different field strengths, the model achieved a recall of 0.89 with a DSC of 0.90 in Siemens 3.0T group and a recall of 0.84 with a DSC of 0.89 in Siemens 1.5T group, no significant difference being found between the 2 groups (Z = 1.77 and 0.23; p = 0.08 and 0.82, respectively). In the diagnostic accuracy comparison trial, the model exhibited a sensitivity of 72.0% and a FP per patient of 0.94, which were both higher than those of the resident radiologists and the attending radiologists (a sensitivity of 48.5% and 68.1% with the FP per patient of 0.13 and 0.32, respectively). For single tiny BM, reading time of the model was significantly shorter than that of radiologists (2.29 ± 0.45 sec/case vs. 21.53 ± 0.64 sec/case, Z = -6.15, p < 0.01). For isolated BM with different diameters, the average time for the model to segment each lesion were 2.27 ± 0.35 s for 10mm group and 2.30 ± 0.59 s for 20mm group, both of which were shorter than that of radiologists (Z = -5.09 and -4.78, all p <0.001).Discussion and Conclusion
The present study established a specific deep learning model to automatically detect and segment brain metastases on contrast-enhanced T1WI images. The results not only demonstrated the model’s good performance, but also verified the model’s high robustness and generalizability. In addition, comparison tests with radiologists showed the model’s higher sensitivity and efficiency, as expected. Afore-mentioned results suggested that the proposed model is a promising tool to assist the workflow in the clinical scenario.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 8212018014, 82071908, and 82101998), Sichuan Science and Technology Program (Grant Nos. 2021JDTD0002 and 2020YJ0018), the Science and Technology Project of the Health Planning Committee of Sichuan (Grant No. 20PJ010), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project No. ZYYC08001 and ZYJC18020). SL also acknowledges the support from Humboldt Foundation Friedrich Wihelm Bessel Research Award and Chang Jiang Scholars (Program No. T2019069).References
1. Suh JH, Kotecha R, Chao ST, et al. Current approaches to the management of brain metastases. Nat Rev Clin Oncol, 2020;17(5):279-299.
2. Owonikoko TK, Arbiser J, Zelnak A, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol, 2014;11(4):203-222.
3. Kaal EC, Niël CG, Vecht CJ. Therapeutic management of brain metastasis. Lancet Neurol, 2005;4(5):289-298.
4. Carden CP, Agarwal R, Saran F, et al. Eligibility of patients with brain metastases for phase I trials: time for a rethink? Lancet Oncol, 2008;9(10): 1012-1017.
5. Olson JJ, Kalkanis SN, Ryken TC. Congress of neurological surgeons systematic review and evidence-based guidelines for the treatment of adults with metastatic brain tumors: Executive summary. Neurosurgery, 2019; 84(3): 550-552.
6. Zhou Z, Sanders JW, Johnson JM, et al. Computer-aided Detection of Brain Metastases in T1-weighted MRI for Stereotactic Radiosurgery Using Deep Learning Single-Shot Detectors. Radiology, 2020;295(2):407-415.
7. Noguchi T, Uchiyama F, Kawata Y, et al. A Fundamental Study Assessing the Diagnostic Performance of Deep Learning for a Brain Metastasis Detection Task. Magn Reson Med Sci, 2020;19(3):184-194.
8. Bousabarah K, Ruge M, Brand JS, et al. Deep convolutional neural networks for automated segmentation of brain metastases trained on clinical data. Radiat Oncol, 2020;15(1):87.
9. Dikici E, Ryu JL, Demirer M, et al. Automated Brain Metastases Detection Framework for T1-Weighted Contrast-Enhanced 3D MRI. IEEE J Biomed Health Inform, 2020;24(10):2883-2893.