3735
Diffusion kurtosis imaging predicts positive margins after radical resection of localized prostate cancer1Radiology, The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2The First Affiliated Hospital of Dalian Medical University, Dalian, China
Synopsis
PSMs are an independent risk factor for biochemical recurrence in patients after radical prostatectomy. DKI quantifies the non-Gaussian nature of the real water molecule diffusion, and accurately reveals the changes in the microstructure of the tissue. Results of this study indicate that MD, Da , Dr, MK, Ka and Kr can be used to predict predicts positive margins after radical resection of localized prostate cancer.
Introduction
Radical prostatectomy has been widely used in clinical practice and is currently the main way to treat localized prostate cancer (PCa) [1]. Among patients undergoing radical prostatectomy, 6%–45.7% had positive surgical margins (PSMs) [2-4]. PSMs are an independent risk factor for biochemical recurrence in patients after radical prostatectomy [5, 6]. Therefore, the prediction of PSMs is of great value for evaluating the prognosis of PCa patients. Diffusion kurtosis imaging (DKI) fully considers the influence of the microstructure of the tissue on the diffusion process of water molecules, quantifies the non-Gaussian nature of the real water molecule diffusion, and accurately reveals the changes in the microstructure of the tissue [7]. And it has high clinical application value in the diagnosis of PCa [8]. Therefore, preoperative DKI evaluation can help clinicians accurately determine which patients are most likely to benefit from radical prostatectomy and reduce the positive rate of resection margins. The purpose of this study was to find DKI imaging markers related to PSMs, and to provide a non-invasive visualization method for predicting PSMs before surgery.Methods
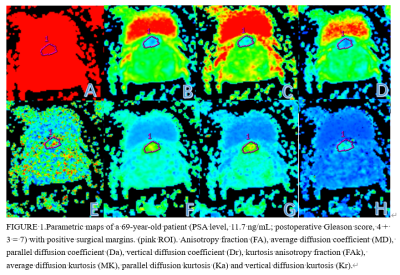
All PCa patients from January 2018 to December 2019, who recruited DKI sequences that received 3.0T MR scans, and received radical prostatectomy. Patients with 1) receiving endocrine therapy before surgery; 2) incomplete MRI sequence acquisition. 3) the lesion cannot be displayed on the MRI image 4) the pathological margin is not assessed and uncertain. Finally, 40 PCa patients were included in this study. Age ranged from 59 to 81 years, mean age 70±7 years. A 3.0T MR scanner (Signa HDxt, General Electric) and an 8-channel phased-array surface coil were used. DKI was acquired separately using a single-shot echo-planar imaging pulse sequence with the following imaging parameters: TR, 2500 milliseconds; TE, 80 milliseconds; FOV , 350×350 mm2; matrix, 128×128; slice thickness, 7mm; and b values, 0, 1000 and 2000 s/mm2. 15 orthogonal diffusion directions were acquired, and trace-weighted images were calculated. The total acquisition time was 2 minutes and 58 seconds. The quantitative analysis of DKI is to use diffusion analysis software (DKI, General Electric) to calculate statistics of the regions of interest (ROIs) of average diffusion kurtosis (MK), parallel diffusion kurtosis (Ka), vertical diffusion kurtosis (Kr), kurtosis anisotropy fraction (FAk), average diffusion coefficient (MD), parallel diffusion coefficient (Da), vertical diffusion coefficient (Dr) and anisotropy fraction (FA). The region of interest (ROI) was placed on the largest slice of the tumor, and contained the whole tumor as much as possible (Figure 1). According to the PSMs, the PCa patients were individed to two group: PSMs group and negative surgical margins (NSMs) group. The intraclass correlation coefficient (ICC) was used to test the consistency of the two observers. The differences between groups were analyzed by Mann-Whitney U test and independent sample T test. Receiver-operating characteristic (ROC) curves to distinguish PSMs group from negative margin group. The diagnostic potential was determined by calculating the area under the curve (AUC). Youden Index (Youden index = sensitivity + specificity - 1) was calculated and used for determining threshold values. Significance level of all ROC analyses was tested according to the DeLong test.Results
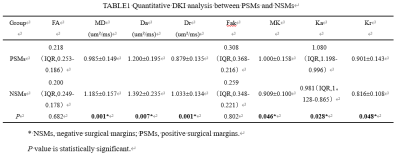
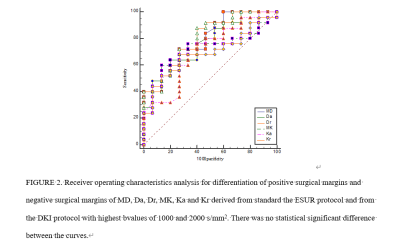
Inter-observer repeatability agreement was excellent in the orbital mass for all the MRI parameters (ICC≥75%). A comparison of DKI parameters between groups was shown in Table 1. The MD, Da and Dr of PSMs group were significantly lower than those of NSMs group (P≤0.05). The MK, Ka and Kr of PSMs group were significantly higher than those of NSMs group (P≤0.05). There was no statistical difference in FA and Fak. The sensitivity, specificity and AUC of the Ktrans were shown in Table 2 and Figure 2. With DeLong test, MD, Da, Dr, MK, Ka and Kr differential diagnosis of PSMs and NSMs in the AUC were not statistically different (P>0.05).Discussion
In our study, the predict value of DKI for PSMs were analyzed. According to the results of the study, MD, Da, Dr, MK, Ka and Kr can be used to predict PSMs. Previous studies have mostly focused on the distinction between PCa and benign prostatic hyperplasia (BPH). The results showed that compared with BHP, prostate cancer had lower MD values, and higher MK values [9]. The results of our study are similar to it, indicating that DKI can not only differentially diagnose PCa, but also has certain value in predicting PSMs. This may be because DKI can obtain both the diffusion index measurement and the diffusion kurtosis measurement, which reflects the overall diffusion level and diffusion resistance of water molecules in the tissue, and provides more information for evaluating tumor heterogeneity [10]. Compared with NSMs lesions, tumor cell proliferation is more obvious in PSMs lesions, and the arrangement of tumor spatial microstructure changes is more complex, so the diffusion index measurement (MD, Da and Dr) is reduced, and the diffusion kurtosis measurement (MK, Ka and Kr) is increased. The quantitative parameters of DKI can evaluate the microarchitecture changes of cells and stromal cells. Then assess the aggressiveness of the tumor. This may affect the prognosis of PCa patients undergoing radical surgery.Conclusion
DKI had the potential to evaluate PCa microenvironment and predict PSMs after radical resection of localized PCa.Acknowledgements
No acknowledgement found.References
[1] Miocinovic R, Berglund RK, Stephenson AJ, Jones JS, Fergany A, Kaouk J,Klein EA. Avoiding androgen deprivation therapy in men with high-risk prostate cancer: the role of radical prostatectomy as initial treatment.Urology. 2011;77:946–50.
[2] Hsu M, Chang SL, Ferrari M, Nolley R, Presti JC Jr, Brooks JD. Length of sitespecific positive surgical margins as a risk factor for biochemical recurrence following radical prostatectomy. Int J Urol. 2011;18:272–9.
[3] Pettenati C, Neuzillet Y, Radulescu C, Herve JM, Molinie V, Lebret T. Positive surgical margins after radical prostatectomy: what should we care about ? World J Urol. 2015;33:1973–8.
[4] Zhang L, Wu B, Zha Z, Zhao H, Jiang Y, Yuan J. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: a meta-analysis from high-quality retrospective cohort studies. World J Surg Oncol. 2018 Jul 3;16(1):124.
[5] Zhang L, Wu B, Zha Z, Zhao H, Jiang Y, Yuan J. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: a meta-analysis from high-quality retrospective cohort studies. World J Surg Oncol. 2018 Jul 3;16(1):124.
[6] Celik S, Eker A, Bozkurt İH, Bolat D, Basmacı İ, Şefik E, Değirmenci T, Günlüsoy B. Factors affecting biochemical recurrence of prostate cancer after radical prostatectomy in patients with positive and negative surgical margin. Prostate Int. 2020 Dec;8(4):178-184 .
[7] Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005 Jun;53(6):1432-40.
[8] Wang X, Tu N, Qin T, Xing F, Wang P, Wu G. Diffusion Kurtosis Imaging Combined With DWI at 3-T MRI for Detection and Assessment of Aggressiveness of Prostate Cancer. AJR Am J Roentgenol. 2018 Oct;211(4):797-804.
[9] Yin H, Wang D, Yan R, Jin X, Hu Y, Zhai Z, Duan J, Zhang J, Wang K, Han D. Comparison of Diffusion Kurtosis Imaging and Amide Proton Transfer Imaging in the Diagnosis and Risk Assessment of Prostate Cancer. Front Oncol. 2021 Apr 15; 11:640906.
[10] Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005 Jun;53(6):1432-40.
Figures