3726
Reconstructing a four-dimensional diffusion magnetic resonance imaging atlas of the fetal brain1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 2Department of Radiology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China, 3Zhejiang Lab, Hangzhou, China, 4MR Collaboration, Siemens Healthineers Ltd., Shanghai, China
Synopsis
Diffusion MRI (dMRI) is a powerful tool to characterize the developing brain, e.g., to map the spatiotemporal development of early white matter (WM) in the fetal brain and to quantify microstructural changes for diagnosis of prenatal disorders. We proposed a pipeline for dMRI atlas generation with fiber orientation distribution-based registration and shape update, and constructed the first spatiotemporal dMRI atlas of the fetal brain in a Chinese population of 90 normal fetuses from 24 to 38 gestational weeks. The atlas provided information for deciphering the morphological and microstructural development of WM tracts during the second to third trimester.
Introduction
White matter (WM) of the fetal brain undergoes rapid structural and functional development during gestation 1. Previous studies utilized diffusion MRI (dMRI) and the diffusion tensor model to depict WM, reporting increasing fractional anisotropy (FA) and decreasing mean diffusivity (MD) in the fetal brain during the second-to-third trimester 2-4, whereas others reported non-monotonic changes of FA or MD in selected WM regions 5-9. dMRI atlases of the fetal brain are important for characterizing developmental trajectories of WM and providing information for in-utero diagnosis of prenatal abnormalities. To date, the sole fetal brain dMRI atlas generated from in-utero data was constructed based on diffusion tensor only 10 and lacked spatial resolution due to limitations in acquisition and atlas generation pipeline, and it was based on a Caucasian population. The goals of this work were: 1) to develop a fiber orientation distribution-based (FOD-based) registration pipeline for fetal brain dMRI atlas generation; 2) to generate a spatiotemporal dMRI atlas of the Chinese fetal brain; and 3) to decipher the pattern and order of WM development with multi-modal dMRI parameters.Methods
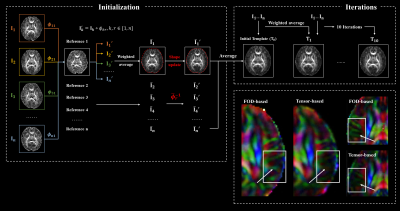
A total of 135 normal fetuses were scanned on a 3T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) using a diffusion-weighted echo-planar imaging sequence with b = 600 s/mm², 30 gradient directions, 8 non-diffusion-weighted images, 1.73 mm in-plane resolution, 4 mm slice thickness, and two averages. We excluded scans with extreme motion, noise, or other abnormalities, resulting in 90 remaining scans acquired at gestational age (GA) 24 to 38 weeks, with 5 to 8 subjects from each week.Raw data were preprocessed in MRtrix3, allowing for denoising, bias removal, and between-volume motion correction 11. Images were slice-to-volume-registered and reconstructed to 1.2 mm isotropic resolution using SVRTK 12. We estimated diffusion tensor imaging (DTI) metrics and FODs, performing fixel-based analysis (FBA) using the reconstructed data. Atlas construction followed an FOD-based pipeline (Figure 1). For each GA, the template was initialized by pair-wise diffeomorphic registrations and averages between subject FODs 13, during which kernel regression in GA was used to calculate the weights of each subject 14. Subject FODs were registered to the template and weighted averages of the co-registered subject images were calculated, iteratively for 10 times to obtain the final FOD atlas. A DTI atlas was obtained by applying the subject-to-atlas warps to diffusion tensors, with tensor reorientation using the finite strain (FS) algorithm 15.
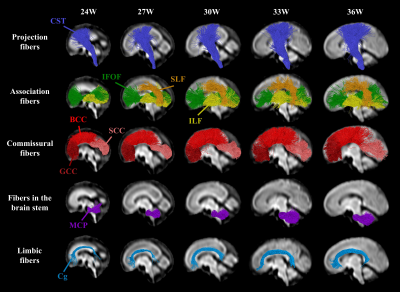
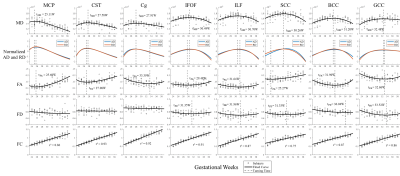
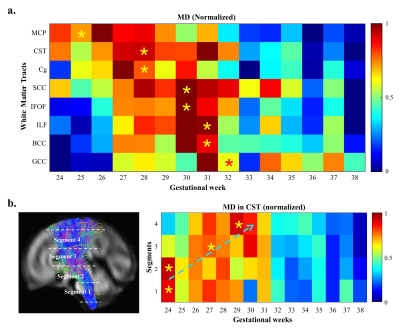
Probabilistic tractography was performed on the FOD atlases to estimate major WM tracts, including the genu, body and splenium of corpus callosum (GCC, BCC, SCC), cortico-spinal tract (CST), cingulum bundle (Cg), inferior longitudinal fasciculus (ILF), inferior fronto-occipital fasciculus (IFOF), and middle cerebellar peduncle (MCP). The tracts were filtered using the Topographic Tract Filtering (TTF) tool 16 and transformed back to subject spaces. Mean DTI- and FBA-based measures along WM tracts were extracted from each subject and fitted to a flexible sigmoid function over GA 17, except for fiber cross-section (FC), which was linearly fitted. Axial diffusivity (AD) and radial diffusivity (RD) were normalized for visualization. We extracted MD values in four segments of CST to study the along-tract variability (Figure 5b).
Results
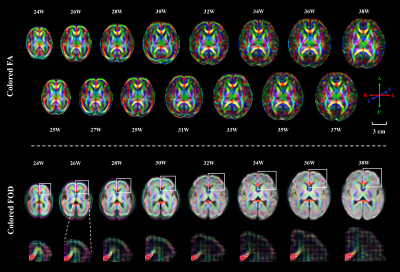
A spatiotemporal fetal brain dMRI atlas was generated at 24 to 38 weeks’ GA using the proposed pipeline, in both DTI and FOD spaces (Figure 2). With our FOD-based pipeline, the population-average atlas demonstrated shaper structural details in cortical regions compared to the existing pipeline with tensor-based registration (Figure 1) 10, indicating better registration accuracy. Eight WM tracts were successfully traced (Figure 3). The mean FA values of WM tracts decreased until around 30 weeks then increased toward 38 weeks, whereas MD changed in an inversed trend. AD and RD showed trends similar to MD, but their relative changes varied over GA (Figure 4). The turning points fitted with sigmoid function were statistically significant (p < 0.05) in FA and MD trajectories. FC increased significantly over GA (r² ≥ 0.75) in all tracts, whereas fiber density (FD) first decreased before increasing or had insignificant change (Figure 4).Discussion
Although oligodendrocytes (OLs) begin to proliferate and differentiate in the second trimester, active myelinogenesis does not start due the “arrested” state of immature OLs 18. The decreasing FA and increasing MD before the turning-points in the developmental trajectories may result from the quick volumetric growth (indicated by increasing FC) and relatively slow axonal organization processes (indicated by decreasing FD) in WM 4,6, when RD increases faster than AD. During the late third trimester, when pre-myelination starts 6,18,19, WM microstructures mature rapidly, possibly leading to a more rapid decrease of RD than AD, resulting in the decreasing MD and increasing FA. Therefore, we hypothesize that the turning-points of MD may suggested the starting-times of pre-myelination (Figure 5a), which agreed with the developmental order reported in previous MRI and the histological studies 20-22. The maturation order long the CST also agreed with the previously reported proximal-to-distal order of WM myelination (Figure 5b) 23.Conclusion
We generated the first Chinese fetal brain dMRI atlas using an FOD-based pipeline. The atlas provided rich information for deciphering the developmental patterns of major WM tracts, e.g., the temporal order of pre-myelination in the fetal brain.Acknowledgements
This work is supported by the Ministry of Science and Technology of the People’s Republic of China (2018YFE0114600), National Natural Science Foundation of China (61801424, 81971606, 82122032), and Science and Technology Department of Zhejiang Province (202006140).References
1. Huang H, Zhang J, Wakana S, et al. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33(1): 27-38.
2. Bui T, Daire J-L, Chalard F, et al. Microstructural development of human brain assessed in utero by diffusion tensor imaging. Pediatric Radiology. 2006;36(11): 1133-1140.
3. Jiang S, Xue H, Counsell S, et al. Diffusion Tensor Imaging (DTI) of the Brain in Moving Subjects: Application to In-Utero Fetal and Ex-Utero Studies. Magnetic Resonance in Medicine. 2009;62(3): 645-655.
4. Jaimes C, Machado-Rivas F, Afacan O, et al. In vivo characterization of emerging white matter microstructure in the fetal brain in the third trimester. Human Brain Mapping. 2020;41(12): 3177-3185.
5. Schneider J F, Confort-Gouny S, Le Fur Y, et al. Diffusion-weighted imaging in normal fetal brain maturation. European Radiology. 2007;17(9): 2422-2429.
6. Zanin E, Ranjeva J-P, Confort-Gouny S, et al. White matter maturation of normal human fetal brain. An in vivo diffusion tensor tractography study. Brain and Behavior. 2011;1(2): 95-108.
7. Hooker J D, Khan M A, Farkas A B, et al. Third-trimester in utero fetal brain diffusion tensor imaging fiber tractography: a prospective longitudinal characterization of normal white matter tract development. Pediatric Radiology. 2020;50(7): 973-983.
8. Wilson S, Pietsch M, Cordero-Grande L, et al. Development of human white matter pathways in utero over the second and third trimester. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(20):
9. Machado-Rivas F, Afacan O, Khan S, et al. Spatiotemporal changes in diffusivity and anisotropy in fetal brain tractography. Human Brain Mapping. 2021;
10. Khan S, Vasung L, Marami B, et al. Fetal brain growth portrayed by a spatiotemporal diffusion tensor MRI atlas computed from in utero images. Neuroimage. 2019;185(593-608.
11. Tournier J D, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202(
12. Deprez M, Price A, Christiaens D, et al. Higher Order Spherical Harmonics Reconstruction of Fetal Diffusion MRI With Intensity Correction. Ieee Transactions on Medical Imaging. 2020;39(4): 1104-1113.
13. Serag A, Aljabar P, Ball G, et al. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage. 2012;59(3): 2255-2265.
14. Davis B C, Fletcher P T, Bullitt E, et al. Population Shape Regression from Random Design Data. International Journal of Computer Vision. 2010;90(2): 255-266.
15. Alexander D C, Pierpaoli C, Basser P J, et al. Spatial transformations of diffusion tensor magnetic resonance images. Ieee Transactions on Medical Imaging. 2001;20(11): 1131-1139.
16. Wang J, Aydogan D B, Varma R, et al. Modeling topographic regularity in structural brain connectivity with application to tractogram filtering. Neuroimage. 2018;183(87-98.
17. Yin X Y, Goudriaan J, Lantinga E A, et al. A flexible sigmoid function of determinate growth (vol 91, pg 361, 2003). Annals of Botany. 2003;91(6): 753-753.
18. Back S A, Luo N L, Borenstein N S, et al. Arrested oligodendrocyte lineage progression during human cerebral white matter development: Dissociation between the timing of progenitor differentiation and myelinogenesis. Journal of Neuropathology and Experimental Neurology. 2002;61(2): 197-211.
19. Dubois J, Dehaene-Lambertz G, Kulikova S, et al. THE EARLY DEVELOPMENT OF BRAIN WHITE MATTER: A REVIEW OF IMAGING STUDIES IN FETUSES, NEWBORNS AND INFANTS. Neuroscience. 2014;276(48-71.
20. Brody B A, Kinney H C, Kloman A S, et al. SEQUENCE OF CENTRAL-NERVOUS-SYSTEM MYELINATION IN HUMAN INFANCY .1. AN AUTOPSY STUDY OF MYELINATION. Journal of Neuropathology and Experimental Neurology. 1987;46(3): 283-301.
21. Huang H and Vasung L. Gaining insight of fetal brain development with diffusion MRI and histology. International Journal of Developmental Neuroscience. 2014;32(11-22.
22. Ouyang M, Dubois J, Yu Q, et al. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage. 2019;185(836-850.
23. Kinney H C V, Joseph J. Chapter 8 - Myelination Events. Volpe's Neurology of the Newborn (Sixth Edition). 6. 2018.
Figures