3725
Susceptibility-Weighted Imaging for Evaluation of Fetal Vertebrae and Vertebral Anomalies1Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China; Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China., Jinan, China, China, 2Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China., Jinan, China, China, 3MR Collaboration, Siemens Healthineers Ltd, Beijing, China. Email: jinxia.zhu@siemens-healthineers.com, beijing, China
Synopsis
This study explored the feasibility and clinical value of susceptibility weighted imaging (SWI) in depicting fetal vertebral growth and related anomalies. The results showed that vertebral development in fetuses on SWI was remarkably linearly correlated with gestational ages. In addition, SWI demonstrated superior image quality and higher diagnostic accuracy compared to conventional Half-Fourier acquisition single-shot turbo spin-echo (HASTE) or true fast imaging with steady-state precession (TrueFISP) sequences. In conclusion, SWI is a reliable choice when imaging fetal vertebrae and vertebral anomalies.
Introduction
Congenital anomalies of the spine result from abnormal vertebral development during weeks 4 to 6 of gestation 1, often leading to asymmetric spinal growth. Spine anatomy imaging and its relevant pathology is clinically important for early identification of spinal malformations and anomalous osseous development. Ultrasonography (US) is the mainstay for evaluating fetal anatomy and malformations before birth 2. However, issues such as limited US wave penetration in conditions like maternal obesity, presence of bony structures, abnormal fetal position, and oligohydramnios could preclude proper US examinations 3.Fetal MR imaging is well-established as a powerful tool for prenatal evaluation of the neuroaxis and plays an important role in prenatal diagnosis. Half-Fourier acquisition single-shot turbo spin-echo (HASTE) or true fast imaging with steady-state precession (TrueFISP) are widely used as valuable adjuncts to US to visualize fetal spinal canal and spinal cord pathologies. However, these sequences are not ideal for prenatal diagnosis of osseous malformations and vertebral development.
Susceptibility-weighted imaging (SWI) is a fully flow-compensated sequence that can be used to visualize microbleeds and changes in venous oxygenation levels 4, 5 and has recently been modified for fetal imaging 6. In this study, we adapted a clinically available SWI sequence for fetal spine imaging and evaluated its performance compared to HASTE and TrueFISP sequences.
Materials and Methods
Ninety-seven women with normal fetal vertebra and 127 women with suspected vertebral anomalies on ultrasound who underwent MR examinations were included. The MRI protocols were performed on a 1.5T MR system (MAGNETOM Amira, Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China) including SWI, TrueFISP, and HASTE. The imaging parameters were as follows (1) HASTE: TR/TE=1300/93 ms; flip angle=180º; FOV=380×308.8 mm2; matrix=256×198; slice thickness=4.0 mm; voxel size=1.5×1.5×4.0 mm3; acquisition time=21s; in a free breathing manner. (2) TrueFISP: TR/TE=4.06/1.76 ms; flip angle=79°; FOV=380×310 mm2; matrix=304×198; slice thickness=4.0 mm; voxel size=1.3×1.3×4.0 mm3; acquisition time=12 s; and free breathing. (3) SWI: TR/TE=85/12.40 ms; flip angle=15°; FOV=300×244.8 mm2; matrix=256×166; slice thickness=3.0 mm; reconstructed voxel size =0.6×0.6×3.0 mm3; acquisition time=26 s; images were obtained in two breath-holds, 13s each. The image quality between HASTE/TrueFISP and SWI was compared using the paired t test, and the following parameters were measured on SWI to investigate the correlation with gestational age: height, transverse, sagittal diameter, and area of ossification center at L1 centrum. The diagnostic performance of HASTE/TrueFISP and SWI regarding fetal vertebral anomalies was performed using the Chi-square test or Fisher exact test and the area under curve (AUC) of the receiver-operating characteristic (ROC) curves was also calculated.Results
The overall visibility of fetal vertebrae structures in SWI (3.58±0.69) was significantly greater than HASTE (1.98±0.51, P < 0.001) or TrueFISP (2.63±0.52, P < 0.001), and the same was true for cervical, thoracic, and lumbosacral structures (all P < 0.001) (Figure 1, 2). The height, transverse, sagittal diameter, and area of ossification center at L1 centrum were linearly correlated with gestational age (all P < 0.001) (Figure 3). For the 127 cases with suspected fetal vertebral anomalies, the diagnostic accuracy of SWI (89.0%) was superior to HASTE/TrueFISP (48.0%) (P < 0.001) as was AUC (0.909, 95% CI 0.854, 0.963; P < 0.001) (Table 1). Presentative images of fetuses with different vertebral anomalies were shown in Figure 4.Discussion
Although previously the fetal skeleton was observed on T1-weighted gradient-recalled echo images 7, single-shot fast spin-echo or steady-state free precision images 8-10, echo-planar images 11, 12, and thick-slab T2-weighted imaging 13, to our knowledge, this is the first time that SWI was able to characterize normal fetal vertebral ossification growth in vivo. Previously, growth dynamics of the lumbar spine were reported to be linear 14, 15, quadratic 16, exponential 17, or logarithmically 18 and most were studied postmortem 19. This study showed that growth and development of L1 centrum ossification center in height (R2=0.85), transverse diameter (R2=0.91), sagittal diameter (R2=0.83), and area (R2=0.91) had a clear linear relationship with gestational age (GA).We further showed that SWI outperformed HASTE/TrueFISP in imaging visibility and diagnostic accuracy in depicting fetal vertebra structure and related anomalies. Fetal imaging using SWI has natural advantages on air-or bone-tissue interface which affects the SWI image quality in adults, but is not a prenatal problem for those air-filled structures in adults are filled with fluid in the fetus. Although both TrueFISP and HASTE possess excellent contrast between different soft tissues, they show details of bone and calcified tissues poorly. This is because they have either very short echo times and lack T2* dephasing of the bone marrow or have a long echo time but with a spine echo like acquisition and no T2* dephasing.
Conclusion
SWI is a reliable method for depicting fetal vertebrae structure and growth, which is able to significantly improve diagnostic performance for detection of vertebral anomalies in fetuses.Acknowledgements
noneReferences
1. Giampietro PF, Blank RD, Raggio CL, Merchant S, Jacobsen FS, Faciszewski T, Shukla SK, Greenlee AR, Reynolds C, Schowalter DB. Congenital and idiopathic scoliosis: clinical and genetic aspects. Clin Med Res 2003; 1: 125-136.
2. Garne E, Loane M, Dolk H, De Vigan C, Scarano G, Tucker D, Stoll C, Gener B, Pierini A, Nelen V, Rosch C, Gillerot Y, Feijoo M, Tincheva R, Queisser-Luft A, Addor MC, Mosquera C, Gatt M, Barisic I. Prenatal diagnosis of severe structural congenital malformations in Europe. Ultrasound Obstet Gynecol 2005; 25: 6-11.
3. Hendler I, Blackwell SC, Bujold E, Treadwell MC, Mittal P, Sokol RJ, Sorokin Y. Suboptimal second-trimester ultrasonographic visualization of the fetal heart in obese women: should we repeat the examination? J Ultrasound Med 2005; 24: 1205-1209; quiz 1210-1201.
4. Reichenbach JR, Jonetz-Mentzel L, Fitzek C, Haacke EM, Kido DK, Lee BC, Kaiser WA. High-resolution blood oxygen-level dependent MR venography (HRBV): a new technique. Neuroradiology 2001; 43: 364-369.
5. Haacke EM, Tang J, Neelavalli J, Cheng YC. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imaging 2010; 32: 663-676.
6. Robinson AJ, Blaser S, Vladimirov A, Drossman D, Chitayat D, Ryan G. Foetal "black bone" MRI: utility in assessment of the foetal spine. Br J Radiol 2015; 88: 20140496.
7. Levine D, Hatabu H, Gaa J, Atkinson MW, Edelman RR. Fetal anatomy revealed with fast MR sequences. AJR Am J Roentgenol 1996; 167: 905-908.
8. Chauvin NA, Victoria T, Khwaja A, Dahmoush H, Jaramillo D. Magnetic resonance imaging of the fetal musculoskeletal system. Pediatr Radiol 2020; 50: 2009-2027.
9. Gilligan LA, Calvo-Garcia MA, Weaver KN, Kline-Fath BM. Fetal magnetic resonance imaging of skeletal dysplasias. Pediatr Radiol 2020; 50: 224-233.
10. MR imaging of the fetal musculoskeletal system. Prenat Diagn 2012; 32: 205-213.
11. Ursula Nemec MSFN, MD Michael Weber, PhD Peter C. Brugger, MD, PhD Gregor Kasprian, MD Dieter Bettelheim, MD David L. Rimoin, MD, PhD Ralph S. Lachman, MD Gustavo Malinger, MD Daniela Prayer, MD. <human long Bone Development in Vivo Analysis of the Distal Femoral Epimetaphysis on MR Images of Fetuses.pdf>. Radiology 2013; 267.
12. Nemec SF, Kasprian G, Brugger PC, Bettelheim D, Amann G, Nemec U, Rotmensch S, Graham JM, Jr., Rimoin DL, Lachman RS, Prayer D. Abnormalities of the upper extremities on fetal magnetic resonance imaging. Ultrasound Obstet Gynecol 2011; 38: 559-567.
13. Brugger PC, Mittermayer C, Prayer D. A new look at the fetus: thick-slab T2-weighted sequences in fetal MRI. Eur J Radiol 2006; 57: 182-186.
14. Margolis AJ, Voss RG. A method for radiologic detection of fetal maturity. Am J Obstet Gynecol 1968; 101: 383-389.
15. Zhang S, Yuan X, Peng Z, Jian N, Tian M, Feng X, Lin X, Wang X. Normal fetal development of the cervical, thoracic, and lumbar spine: A postmortem study based on magnetic resonance imaging. Prenat Diagn 2021; 41: 989-997.
16. Bagnall KM, Harris PF, Jones PR. A radiographic study of the human fetal spine. 3. Longitudinal growth. Journal of anatomy 1979; 128: 777-787.
17. Schild RL, Wallny T, Fimmers R, Hansmann M. The size of the fetal thoracolumbar spine: a three-dimensional ultrasound study. Ultrasound Obstet Gynecol 2000; 16: 468-472.
18. Szpinda M, Baumgart M, Szpinda A, Woźniak A, Mila-Kierzenkowska C. New patterns of the growing L3 vertebra and its 3 ossification centers in human fetuses - a CT, digital, and statistical study. Medical science monitor basic research 2013; 19: 169-180.
19. Matsubara Y, Higaki T, Tani C, Kamioka S, Harada K, Aoyama H, Nakamura Y, Akita T, Awai K. Demonstration of Human Fetal Bone Morphology with MR Imaging: A Preliminary Study. Magn Reson Med Sci 2020; 19: 310-317.
Figures
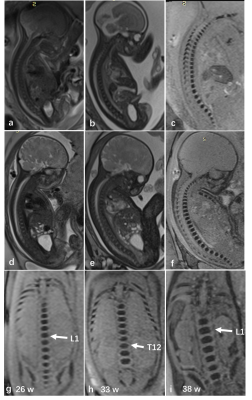
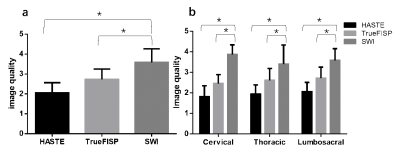
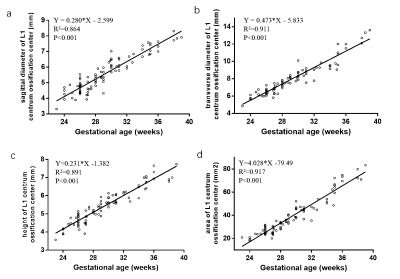
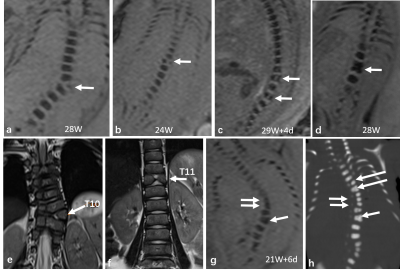
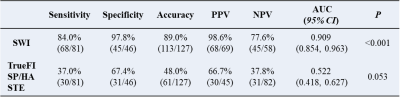
Table 1 Diagnostic performance analysis for SWI and HASTE/TrueFISP
AUC, Area Under Curve; HASTE, Half-Fourier Acquisition Single-shot Turbo spin-echo; NPV, negative predictive value; PPV, Positive Predictive Value; SWI, Susceptibility-weighted imaging: TrueFISP, true fast imaging with steady state precession.