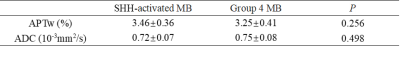3721
Distinguishing sonic hedgehog from Group 4 medulloblastoma using amide proton transfer–weighted MR Imaging1Radiology, ZhuJiang Hospital of Southern Medical University, GuangZhou, China, 2Philips Healthcare, GuangZhou, China
Synopsis
Preoperative prediction of molecular subgroup based on magnetic resonance imaging (MRI) remains a challenge. The objective of this study was to assess amide proton transfer-weighted (APTw) method in medulloblastoma (MB). Preliminary results showed that the mean value of APT value in sonic hedgehog (SHH) -activated MB tended to be higher than that of Group 4 MB.
INTRODUCTION
Medulloblastoma (MB) is one of the most common malignant pediatric brain tumors1. Four molecular subgroups of MB are named as WNT, sonic hedgehog (SHH), Group 3 and Group 4, each of which is associated with genetic, transcriptomic, demographic, and prognostic differences2,3. Magnetic resonance imaging (MRI) is the modality of choice to assess pediatric central nervous system (CNS) diseases. However, there is still a lack of effective method to predict molecular subgroups using conventional MRI sequences4,5. Amide proton transfer-weighted (APTw) imaging is a molecular MRI technique that generates image contrast based on endogenous cellular proteins and peptides6. In this study, we aimed to investigate the correlation between APTw and molecular subgroups of MB.METHODS
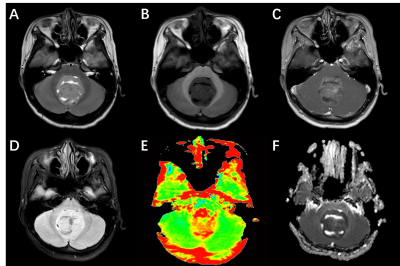
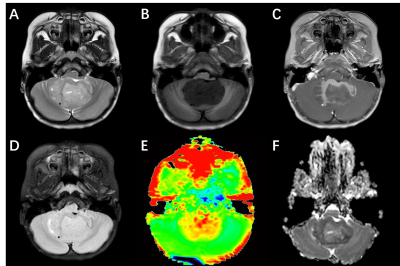
Six patients suspected of MB were recruited to undergo preoperative MRI between Jun. 2021 and Sep. 2021 using a 3.0 T MR scanner (Ingenia, Philips, Best The Netherlands). In addition to anatomic sequences, each MRI consists of DWI and APTw. Molecular classification was based on targeted mutational and chromosomal copy-number variant (CNV) analysis (Genetron Health, China; Genomicare, China, n = 2), NanoString (Simcere, China, n = 2). The APTw parameters in the solid tumor region were obtained and compared between SHH and Group 4 using the Student’s t-test. All image post-processing was performed using Philips DICOM Viewer (version R3.0 SP15). Statistical analysis was performed using software SPSS 24.0. One experienced neuroradiologist analyzed the conventional and APTw images. Regions of interests (ROIs) were distributed based on T2WI. Necrosis, cystic cavities, large vessels, calcification and hemorrhagic components were excluded. The ROIs were co-registered to APT and ADC images. The APTw value obtained from each slice of the tumor was considered as one sample.RESULTS
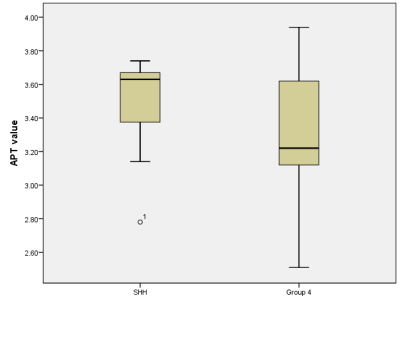
Four patients were pathologically diagnosed as MB (1 male, 3 females; age range, 2-11 years; mean age, 6±3.9 years) including 1 SHH-activated and TP53-wildtype MB, 3 Group 4 MB. A total of 24 samples of tumor were analyzed, 7 from the SHH-activated MB and 17 from the Group 4 MB. The values of the APTw and ADC parameters for the two groups are summarized in Fig. 1. and Fig. 2. The APTw mean values of SHH-activated MB tend to be higher than that of Group 4 MB, but no significant differences were observed in APTw value (P=0.256) and ADC value (P=0.498) between the two groups.DISCUSSION AND CONCLUSION
Over the past decade, methylome profiling has become the gold standard for subgroup assignment in MB7. Preoperative prediction of molecular subgroup based on MRI can potentially aid in guiding brain tumor therapies. Our results suggest that APTw value may correlate with molecular subgroups of MB. APT value of SHH MB was higher than that of Group 4 MB although no significant differences were observed. This study demonstrated the potential of APTw for predicting SHH and Group 4 MB. As a relatively new molecular imaging technique, APTw may provide a preferred alternative method for predicting molecular subtypes of MB and a larger sample size would be desired for further evaluation.Acknowledgements
No acknowledgement found.References
1. Northcott PA, Robinson GW, Kratz CP, et al. Medulloblastoma. Nat Rev Dis Primers. 2019;5:11.
2. Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465-472.
3. Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131:821-831.
4. Yan J, Liu L, Wang W, et al. Radiomic Features From Multi-Parameter MRI Combined With Clinical Parameters Predict Molecular Subgroups in Patients With Medulloblastoma. Frontiers in Oncology. 2020;10.
5. Dasgupta A, Gupta T, Pungavkar S, et al. Nomograms based on preoperative multiparametric magnetic resonance imaging for prediction of molecular subgrouping in medulloblastoma: results from a radiogenomics study of 111 patients. Neuro-Oncology. 2019;21:115-124.
6. Zhou J, Heo HY, Knutsson L, et al. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging. 2019;50:347-364.
7. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology. 2021;23:1231-1251.
Figures