3708
Comparison between Biparametric and Multiparametric MRI for Detection of Prostate Cancer in the Peripheral Zone using PI-RADS Version 2.11Department of Radiology, Peking Union Medical College Hospital, Beijing, China, 2Peking Union Medical College Hospital, Beijing, China
Synopsis
This study aimed to compare and analyse the diagnostic value of PI-RADS v2.1 when used with bpMRI versus mpMRI, DWI versus T2WI to detect peripheral-zone prostate cancer (pzPCa) and clinically significant prostate cancer (csPCa). The diagnostic efficiencies of mpMRI and bpMRI as well as DWI and T2WI in pzPCa and csPCa were compared using a PI-RADS score of ≥4 as the positive threshold and pathologic results as the gold standards. MpMRI and bpMRI as well as DWI and T2WI using PI-RADS v2.1 exhibited similar diagnostic efficiency in pzPCa and csPCa, whereas bpMRI demonstrated increased diagnostic specificity compared with mpMRI.
Introduction and Purpose
Prostate cancer (PCa) is a common malignant tumour in males. PCa has the highest incidence of all cancers worldwide, is the sixth most common cancer in China 1,2. The Prostate Imaging Reporting and Data System (PI-RADS) is currently recognized as an important guideline for prostate examination and diagnosis via MRI. The association developed the first and second editions of PI-RADS guidelines (v1, v2) in 2012 and 2015, respectively 3,4. In 2019, PI-RADS version 2.1 (v2.1) reformulated the research specifications for MRI acquisition technology in prostate scanning and analysed the diagnostic values of T2WI, DWI, and DCE-MRI 5. In the PI-RADS v2.1 system, DCE is the secondary scoring sequence of the peripheral zone (PZ). When lesions in the prostate peripheral zone are scored as 3 on DWI and the DCE score is positive, the total PI-RADS v2.1 score is increased from 3 to 4 (such as Fig 1). DCE-MRI is used only to evaluate PZ lesions and has no effect on the transitional-zone lesion score. Therefore, some studies have proposed biparametric MRI (bpMRI) without DCE instead 6. Since PI-RADS v2.1 was proposed in 2019, relatively few studies on its accuracy in peripheral-zone prostate cancer (pzPCa) and clinically significant prostate cancer (csPCa) have been conducted 7. Therefore, this study aimed (1) to compare and analyse the diagnostic value of PI-RADS v2.1 between mpMRI and bpMRI for detecting pzPCa and csPCa using prostate biopsy and radical prostatectomy as the gold standard and (2) to explore the diagnostic efficiencies of DWI and T2WI sequences in pzPCa and csPCa.Methods
This retrospective study was approved by the ethics committee of our hospital, and the requirement for informed consent was waived. The clinical and MRI data of 491 patients with prostate diseases admitted to our hospital from January 2015 to September 2018 were continuously collected. The inclusion criteria were as follows: (1) a complete MRI examination, including T1WI, T2WI, DWI, the corresponding apparent diffusion coefficient (ADC) map, and DCE sequence; (2) diagnosis confirmed by prostate biopsy or radical prostatectomy after MRI; and (3) no history of prostate treatment before MRI. The exclusion criteria were as follows: (1) after MRI examination, the lesion was suspected to be located in the central gland; (2) poor MRI image quality affected scoring (n = 5); and (3) biopsy prior to MRI. A 3.0-T, eight-channel, surface-phased array coil abdominal MRI scanning system (GE750; GE Health care, Milwaukee, WI, USA) was used in this study. The scan sequence included horizontal T1WI and T2WI, coronal and sagittal T2WI, fat suppression T2WI, DWI, and DCE. The diagnostic efficiencies of mpMRI and bpMRI as well as DWI and T2WI in pzPCa and csPCa were compared using a PI-RADS score of ≥4 as the positive threshold and prostate biopsy and radical prostatectomy as the gold standards.Results
A total of 307 PCa cases were included in the study, including 142 in the non-pzPCa group, 165 in the pzPCa group, and 130 in the csPCa group. The results were summarized in Tables 1 and 2. The AUCs of mpMRI and bpMRI were 0.717 and 0.733 (P = 0.317), respectively, for the diagnosis of pzPCa (sensitivities: 89.1% and 81.8%; specificities: 54.2% and 64.8%, both P < 0.001) and 0.594 and 0.602 (P = 0.756), respectively, for the diagnosis of csPCa (sensitivities: 93.1% and 86.2%, P = 0.004; specificities: 25.7% and 34.3%, P = 0.250) (Fig 2). The AUCs of DWI and T2WI were 0.733 and 0.749 (P = 0.308), respectively, for the diagnosis of pzPCa (sensitivities: 81.8% and 84.2%; specificities: 64.8% and 66.2%, both P > 0.05) and 0.602 and 0.581 (P = 0.371), respectively, for the diagnosis of csPCa (sensitivities: 86.2% and 87.7%; specificities: 34.3% and 28.6%, both P > 0.05) (Fig 3).Discussion and conclusions
With the promotion of PSA screening and the popularization of MRI, the demand for prostate MRI examinations is increasing. The advantages of bpMRI in shortening the scanning time and reducing the examination cost are becoming better known. Therefore, exploring the application value of bpMRI in the diagnosis of PCa has important clinical significance. MpMRI and bpMRI using PI-RADS v2.1 exhibited similar diagnostic efficiency in pzPCa and csPCa. BpMRI using PI-RADS v2.1 with higher diagnostic specificity can potentially help avoid unnecessary biopsies when clinical information, such as PSA, is also considered. DWI and T2WI sequences exhibited similar diagnostic performances in pzPCa and csPCa. When the scanning quality of DWI images is poor and subsequently affects the diagnosis of prostate diseases, T2WI sequence can be used as the main sequence of bpMRI.Acknowledgements
We sincerely thank the participants in this study.
References
1. Chen W, Zheng R, Baade P D, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132.
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
3. Barentsz J O, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746-757.
4. Gupta R T, Mehta K A, Turkbey B, et al. PI-RADS: Past, present, and future. J Magn Reson Imaging. 2020;52:33-53.
5. Turkbey B, Rosenkrantz A B, Haider M A, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019;76:340-351.
6. Scialpi M, Rondoni V, Aisa M C, et al. Is contrast enhancement needed for diagnostic prostate MRI? Transl Androl Urol. 2017;6:499-509.
7. Tamada T, Kido A, Yamamoto A, et al. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2.1. J Magn Reson Imaging. 2021;53:283-291.
Figures
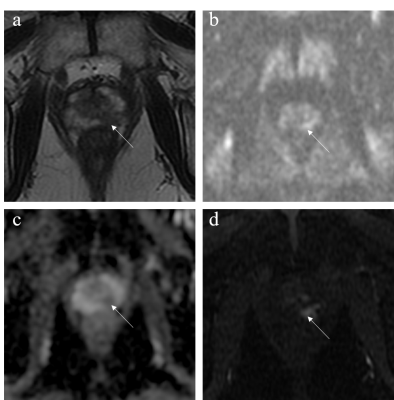
Figure 1. Images from a 71-year-old male patient with a total PSA of 7.57 ng/mL. (a) T2WI score was 4. (b,c) DWI score was 3. (d) DCE score was positive. PI-RADS v2.1 scores were 4 and 3 based on mpMRI and bpMRI, respectively. The lesion was confirmed to be csPCa.
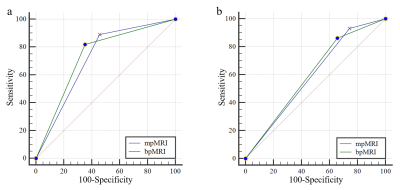
Figure 2. (a) The AUCs of mpMRI and bpMRI in the diagnosis of pzPCa were 0.717 and 0.733, respectively. (b) The AUCs of mpMRI and bpMRI in the diagnosis of csPCa were 0.594 and 0.602, respectively.

Figure 3. (a) The AUCs of DWI and T2WI in the diagnosis of pzPCa were 0.733 and 0.749, respectively. (b) The AUCs of DWI and T2WI in the diagnosis of csPCa were 0.602 and 0.581, respectively.
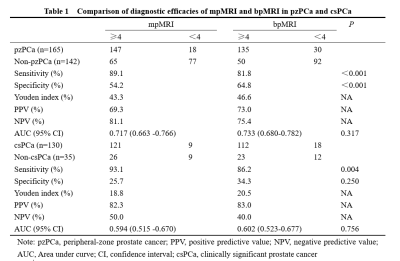
Table 1. Comparison of diagnostic efficacies of mpMRI and bpMRI in pzPCa and csPCa.
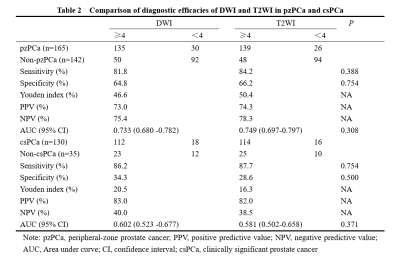
Table 2. Comparison of diagnostic efficacies of DWI and T2WI in pzPCa and csPCa.