3688
DPG-BUDA: Distortion-free diffusion-weighted renal MRI in small rodents1School of Biomedical Engineering, Southern Medical University, Guangzhou, China, 2Guangdong Provincial Key Laboratory of Medical Image Processing & Guangdong Province Engineering Laboratory for Medical Imaging and Diagnostic Technology, Southern Medical University, Guangzhou, China, 3Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence & Key Laboratory of Mental Health of the Ministry of Education, Southern Medical University, Guangzhou, China
Synopsis
We introduced blip-up/down acquisition (BUDA) strategy for interleaved 2-shot EPI to achieve motion robust, distortion-free rat renal diffusion-weighted imaging (DWI). Dual-polarity GRAPPA (DPG) method was implemented to correct Nyquist ghost artifact while simultaneously reconstructing the undersampled 2-shot data, then the field map was estimated from the opposing-distorted images. The field map was incorporated into a joint reconstruction with a structure low-rank constraint across the two shots to eliminate distortion and phase inconsistencies. DPG-BUDA permits high anatomical integrity while holding high scan efficiency for renal DWI in small rodents, it has the potential to benefit the characterization of the renal microstructure.
Introduction
As a non-invasive magnetic resonance imaging (MRI) technique, renal diffusion-weighted imaging (DWI) probes the microstructure integrity and function of renal tissues, which is getting increasingly popular in both pre-clinical and clinical studies1. At an ultra-high magnetic field, single-shot echo-planar imaging (ss-EPI) based renal DWI suffers from severe image distortion which can be mitigated by multi-shot EPI (ms-EPI) variants. Further, to eliminate the distortion, the field map related to B0 inhomogeneity and eddy current in DWI can be derived from blip-up/down acquisition (BUDA) for interleaved 2-shot EPI using FSL toolbox TOPUP2,3, and then can be incorporated into the reconstruction framework. Recently, the propose of BUDA and joint parallel-imaging reconstruction with a structured low-rank constraint framework enables distortion-free and navigator-free high-resolution DWI in the human brain4,5.In this work, we extended this approach to renal DWI in small rodents to examine the feasibility of BUDA to enhance kidney DWI. In current practice, instead of using SENSE + local phase correction (LPC) method, we implemented the dual-polarity GRAPPA (DPG) method to correct the Nyquist ghost while simultaneously reconstructing the undersampled blip-up/down data, which has shown priority to the LPC method6, and we termed this acquisition and reconstruction framework DPG-BUDA.
Methods
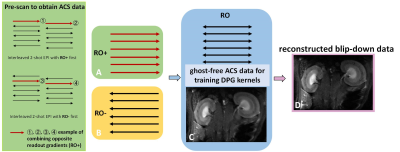
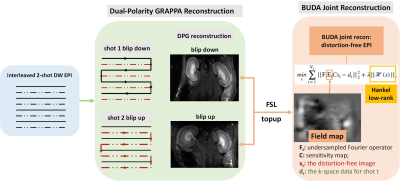
Figure 1 presents the pipeline of the DPG method: two groups of interleaved 2-shot EPI were acquired (with opposing readout gradients, termed RO+ and RO-), the RO+ and RO- readout lines were combined separately, and then temporally encoded ghost-free auto-calibration signals (ACS) were obtained through GESTE7 to train DPG kernels, the undersampled data were reconstructed and corrected through this kernels.Figure 2 presents the framework of the DPG-BUDA acquisition and reconstructed method. Interleaved 2-shot EPI with reversed phase-encoding gradient polarities sampled the k-space. The opposite-distortion images were reconstructed by the DPG method, and by using FSL TOPUP, the field map which contains B0 inhomogeneity and eddy current effects was calculated. The field map was then incorporated into a joint reconstruction with a structure low-rank constraint across 2-shot:
$$min\sum_{t=1}^{N_{s}}||F_{t}E_{t}Cx_{t}-d_{t}||_2^2+\lambda||H(x)||_{*}$$
where Ft is the undersampled Fourier operator in tth shot; Et is the calculated field map; C are the coil-sensitivities; xt is the distortion-free image and dt are the k-space data for shot t. The constraint ||H(x)||* enforces Hankel low-rank prior on the k-space data xt.
This study was approved by the local Institutional Animal Care and Use Committee. All MRI experiments were carried out on a 7.0 T small animal MR scanner (PharmaScan; Bruker BioSpin, Ettlingen, Germany), image reconstructions were performed in Matlab (MathWorks, Natick, MA).
Results
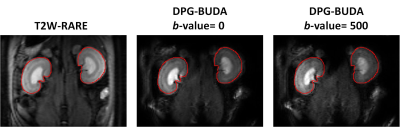
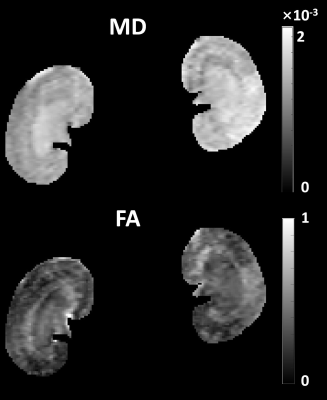
Figure 3 presents representative images from DPG-BUDA method, with both b-value=0 and b-value=500 s/mm2. Both the inter- and intra-structures of renal tissues are clearly depicted by DPG-BUDA. In the left column, the kidney contours were manually segmented for T2W-RARE image, and then were superimposed to the DPG-BUDA images (the medium and the right column of figure 3). Compared with the reference distortion-free T2W-RARE image, the DPG-BUDA yielded high anatomical fidelity.The quantitative mean diffusivity (MD) and fractional anisotropy (FA) maps obtained from DPG-BUDA are displayed in Figure 4. From the FA map, a high degree of diffusion anisotropy in the inner stripe of the outer medulla can be observed.
Discussion and Conclusions
This work demonstrates that renal DWI with high anatomical fidelity can be achieved by the introduced DPG-BUDA acquisition and reconstruction framework. At an ultra-high magnetic field, the spatial resolution of renal DWI is often limited by severe image distortion caused by B0 inhomogeneity and eddy current effects. With a highly efficient interleaved blip-up/down acquisition strategy, the field map can be derived and incorporated into the reconstruction framework to eliminate the distortion. DPG-BUDA method provides high anatomical integrity and high scan efficiency and may has the potential to benefit the study of TE-dependent diffusion imaging to quantify volume fractions of Intra-/inter-tubular water compartments in renal tissues8,9.Acknowledgements
The authors wish to thank Dr. Congyu Liao of Stanford University for generously sharing his BUDA code and Dr. W. Scott Hoge of Harvard Medical School for the inspiration about using DPG reconstruction method and discussion on how to implement it on the Bruker system.References
1. Periquito JS, Gladytz T, Millward JM, et al. Continuous diffusion spectrum computation for diffusion-weighted magnetic resonance imaging of the kidney tubule system. Quant Imaging Med Surg. 2021;11(7):3098-3119.
2. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-888.
3. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. In: Neuroimage. Vol. 23. Cambridge, MA: Academic Press; 2004:S208-S219.
4. Zahneisen B, Aksoy M, Maclaren J, et al. Extended hybrid-space SENSE for EPI: Off-resonance and eddy current corrected joint interleaved blip-up/down reconstruction. Neuroimage. 2017;153:97-108.
5. Liao C, Bilgic B, Tian Q, et al. Distortion‐free, high‐isotropic‐resolution diffusion MRI with gSlider BUDA‐EPI and multicoil dynamic B0 shimming. Magn Reson Med. 2021;86(2):791-803.
6. Hoge WS, Polimeni JR. Dual-polarity GRAPPA for simultaneous reconstruction and ghost correction of echo planar imaging data. Magn Reson Med. 2016;76(1):32-44.
7. Hoge WS, Tan H, Kraft RA. Robust EPI Nyquist ghost elimination via spatial and temporal encoding. Magn Reson Med. 2010;64(6):1781-1791.
8. Ning L, Gagoski B, Szczepankiewicz F, et al. Joint RElaxation-Diffusion Imaging Moments to Probe Neurite Microstructure. IEEE Trans Med Imaging. 2020;39(3):668-677.
9. Veraart J, Novikov DS, Fieremans E. TE dependent Diffusion Imaging (TEdDI) distinguishes between compartmental T2 relaxation times. Neuroimage. 2018;182:360-369.
Figures