3676
Diagnostic performance of peripheral zone prostate cancer: a comparison between PI-RADS v2.1 and PI-RADS v21The Department of Radiology, The General Hospital of Ningxia Medical University, Yinchuan, China, 2GE Healthcare,MR Research, Beijing, China
Synopsis
In this study, we aim to investigate the diagnostic value of PI-RADS v2.1 and PI-RADS v2 (prostate imaging reporting and data system version 2.1 and version 2) for peripheral zone prostate cancer. It was concluded that the diagnostic value of PI-RADS v2.1 was higher.
Summary of Main Findings
PI-RADS system can be used as an important indicator of prostate cancer diagnosis and invasive evaluation, while reducing unnecessary prostate puncture.Introduction
Multi-parametric magnetic resonance imaging (Mp-MRI) is a useful tool for the diagnosis of prostate cancer (PCa), which can provide important clinical information before treatment about histopathological invasiveness and tumor staging[1-2]. The prostate Imaging report and data system (PI-RADS) aims to standardize the imaging report of prostate mp-MRI. In 2012, the first version of standardized prostate MRI guidelines was released [3]. Subsequently, PI-RADSv2 was released in 2015 [4], but it still has some limitations [5-6], such as poor consistency among physicians and ambiguity in scoring criteria. As a result, PI-RADS steering Committee launched an upgraded version of PI-RADS v2 (namely PI-RADS V2.1) in 2019[7]. Although the changes of PI-RADSv2.1 are mainly for transitional zone lesions, it introduces changes to the interpretation of diffusion characteristics, which also affects the evaluation of peripheral zone lesions. Therefore, the purpose of this study was to explore diagnostic value of PI-RADS v2.1 and PI-RADS v2 for peripheral zone Prostate cancer(PCa)and clinically significant prostate cancer(csPCa).Material and Methods
From Jan 2017 to Jun 2020, the clinical and imaging data of 254 patients with lesions located in the peripheral zone(130 cases of prostate cancer, 124 cases of benign prostatic hyperplasia and / or inflammation) confirmed by pathology were analyzed retrospectively in our study. Prostate cancer group: age range 51-89 years old, median age 71 years old, prostate specific antigen (PSA) 0.584-100μg/L, median PSA 42.5μg/L. hyperplasia and / or inflammation group(Benign prostatic lesions group): age range 50-84 years old,median age 66 years old, PSA range 1.35-29.46 μg/L, median PSA 11.98 μg/L. All patients were examined with 3.0T magnetic resonance scanner (Philips 3.0T Ingenia MR, Holland) equipped with 32-channel body phase coil before puncture biopsy or radical operation. The scan sequences included axial T1-weighted、T2-weighted、T2 flair-weighted,sagittal T2-weighted 、coronal T2-weighted imaging ,axial Diffusion-weighted imaging and axial Dynamic enhancement(DCE). Two radiologists were trained by PI-RADS v2.1 and PI-RADS v2 (radiologist 1 and radiologist 2, with 8 and 3 years' experience in prostate diagnosis respectively), who were blinded to the pathological results and PSA levels, independently used PI-RADS v2.1 and PI-RADS v2 to score the peripheral zone lesions. The interval between the first and second scoring by the readers is not less than 4 weeks. Kappa test was used to assess the consistency of the scoring results between two radiologists; ROC curve was used to evaluate and calculate the diagnostic efficiency for PCa and csPCa; Spearman correlation analysis was used to analyze the correlation between the scoring results and Gleason score. Clinically significant prostate cancer (csPCa) was defined as puncture or pathological gross Gleason score ≥ 7 (including 3+4); and / or lesion volume ≥ 0.5cm ³; and / or extracapsular invasion of the prostate.Results
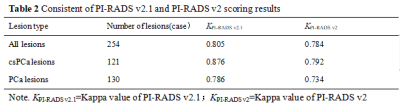
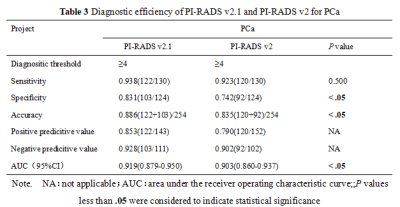
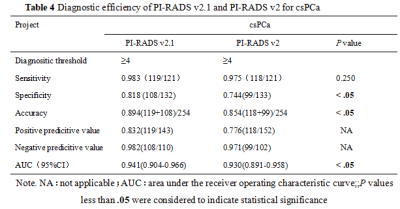
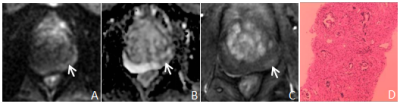
There were 124 cases of benign prostatic hyperplasia and / or inflammation, and 130 cases of PCa, of which 121 cases were csPCa(Table 1). The consistency of PI-RADS v2.1 is better than PI-RADS v2(Table 2). The AUC、specificity and accuracy of PI-RADS v2.1 in diagnosing PCa and csPCa were slightly higher than PI-RADS v2 , and the difference was significant,all P< 0.05. The sensitivity of PI-RADS v2.1 in diagnosing PCa and csPCa were basically similar with PI-RADS v2 ,all P> 0.05. Positive predictive value and negative predictive value of PI-RADS v2.1 in diagnosing PCa and csPCa were slightly higher than PI-RADS v2(Table 3、4,Figure 1). The scoring results of PI-RADS v2.1 and PI-RADS v2 were moderately positively correlated with Gleason score (r=0.585 vs. r=0.559,P <0.05). When PI-RADS 3 score was used as puncture indication, PI-RADS v2 and PI-RADS v2.1 missed 1 case of csPCa respectively.Discussion and Conclusion
Radiologists,consistency of PI-RADS v2.1 was better, in agreement of previous study by Uras et al [8]. The study of Tan Shuangxiu et al [9] showed that the diagnostic efficiency of PI-RADSv2.1 was better, which was consistent with the results of this study. In study of Kim et al [10] the sensitivity of PI-RADSv2.1 in the diagnosis of csPCa was lower than PI-RADSv2.0, which was not consistent with the increased sensitivity of this stud. It may be due to the revised scoring pathway in PI-RADSv2.1 on DWI, resulting in a difference in the proportion of cases with PI-RADS 4 score. The scoring results of PI-RADS v2.1 and PI-RADS v2 were moderately positively correlated with Gleason score, indicating that PI-RADS system is helpful to predict the biological behavior of prostate cancer. One of the major limitations in our study is that the number of samples is too small. Therefore, a large sample study is needed in the future. To conclude, PI-RADS v2.1 has better consistency, higher diagnostic efficiency PI-RADS v2, and the PI-RADS system can guide the puncture and help to detect csPCa.Acknowledgements
We thank wiley for all physicians,assistance of The Department of Radiology, The General Hospital of Ningxia Medical University during the preparation of this manuscript. We thank all of the participants of this study. We also acknowledge the support provided by Dr.Xiaocheng Wei from GE Healthcare. Beijing,China.References
1.Boesen L. Multiparametric MRI in detection and staging of prostate cancer. Dan Med J. 2017;64(2):B5327.
2.MRI imaging and diagnostic consensus of prostate cancer [J]. Chin J radiol,2018,52(10):743-750.
3.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012[J]. European Radiology,2012,22(4):746-757.
4.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging-reporting and data system: 2015, version 2. Eur Urol,2016, 69(1): 16-40.
5.Gupta RT,Mehta KA,Turkbey B, et al. PI-RADS: Past, present, and future.[J] .J Magn Reson Imaging, 2020, 52(1): 33-53.
6.Smith CP, Türkbey B. PI-RADS v2: Current standing and future outlook.Turk J Urol 2018;44(3):189-194.
7.Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imag-ing reporting and data system version 2. Eur Urol 2019;76(3):340-351.
8.Urase Y,Ueno Y,Tamada T, et al. Comparison of prostate imaging reporting and data system v2.1 and 2 in transition and peripheral zones: evaluation of interreader agreement and diagnostic performance in detecting clinically significant prostate cancer.[J] .Br J Radiol, 2021, undefined: 20201434.
9.Comparison of the diagnostic value of prostate imaging reporting and data system version 2 and version 2.1 in the detection of clinically significant prostate cancer [J]. Chinese Journal of Radiology, 2021 Magazine 55 (02): 160-165.
10.Kim HS,Kwon GY,Kim MJ, et al. Prostate Imaging-Reporting and Data System: Comparison of the Diagnostic Performance between Version 2.0 and 2.1 for Prostatic Peripheral Zone.[J] .Korean J Radiol, 2021;22(7):1100-1109.
Figures