3673
Susceptibility-weighted MR sequence for the evaluation of intra-tumoral hemorrhage: Differentiation of benign and malignant ovarian tumors
Mayumi Takeuchi1, Kenji Matsuzaki2, and Masafumi Harada1
1Department of Radiology, Tokushima University, Tokushima, Japan, 2Department of Radiological Technology, Tokushima Bunri University, Sanuki-city, Japan
1Department of Radiology, Tokushima University, Tokushima, Japan, 2Department of Radiological Technology, Tokushima Bunri University, Sanuki-city, Japan
Synopsis
Intra-tumoral hemorrhage is one of the suggestive pathological findings of malignancy. Surgically proven 16 benign and 64 malignant solid or complex ovarian tumors were retrospectively evaluated. High intensity hemorrhagic foci on T1WI were detected in 16 of 64 malignant lesions (25%), whereas signal voids due to hemorrhage on susceptibility-weighted sequence (SWS) were detected in 41 of 64 lesions (64%). Neither high intensity foci on T1WI nor signal voids on SWS was detected in all 16 benign tumors. We conclude that the demonstration of intra-tumoral hemorrhage in patients with ovarian tumors by SWS may provide valuable diagnostic findings.
Introduction
Intra-tumoral hemorrhage is often observed within the solid tissue of malignant tumors due to the breakdown of tumor vasculature, whereas is rarely observed within the solid tissue of benign tumors 1, 2. According to the Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS MRI), T2-weighted signal intensity and diffusion-weighted signal intensity within the solid tissue, and the enhancement of the solid tissue using time intensity curve classification are evaluated to differentiate benign and malignant ovarian tumors 3. We hypothesized that the presence of intra-tumoral hemorrhage may be an another independent factor useful in differentiating benign and malignant ovarian tumors. Intra-tumoral hemorrhage may appear as high signal intensity areas on T1-weighted images, however, the prevalence is not high possibly because only methemoglobin in subacute hemorrhage may show high signal intensity 2. Susceptibility-weighted sequences (SWS) are MR techniques that maximize sensitivity to susceptibility effects and have exquisite sensitivity to blood products such as deoxyhemoglobin and hemosiderin resulting from acute and chronic hemorrhage, respectively 4-7. The purpose of this study was to evaluate the capability of SWS in detecting the signal voids due to intra-tumoral hemorrhage for the differentiation of benign and malignant ovarian tumors.Methods
80 women (mean age 55 years; range 24-89 years) with pathologically proven solid or complex ovarian tumors including 16 benign and 64 malignant, who had undergone MRI examinations including SWS with optimal quality before surgery were retrospectively evaluated. 16 benign tumors included 8 fibromas, 5 thecomas, 2 Brenner tumors and 1 leiomyoma. 64 malignant tumors included 26 serous carcinomas including 23 HGSC, 1 LGSC, and 2 borderline malignancy, 11 clear cell carcinomas, 8 endometrioid carcinomas including 1 borderline malignancy, 2 mucinous carcinomas, 2 malignant Bremmer tumors, 4 malignant germ cell tumors, 3 secondary tumors, and other 8 malignant tumors including 1 borderline malignancy. Gradient echo T1-weighted images with fat saturation, and SWS (SWAN: T2 Star Weighted ANgiography) were obtained for all patients with a 3T (Discovery MR750) or 1.5T (Signa Excite HD or HDx superconducting MRI systems (GE Healthcare) with body-array torso coils. Two radiologists with over 20 years of experience in body MRI qualitatively evaluated the images for the presence of signal voids due to hemorrhage within the solid tissue of the mass on SWS and of high signal intensity hemorrhagic foci within the solid tissue of the mass on fat-saturated T1-weighted images. Signal voids on SWS due to calcification were excluded by referring corresponding phase images and/or CT images. Flow voids of dilated blood vessels were excluded by their curved-linear shape and continuity in multiple slices. The reviewers examined all MR images of the cases independently and then resolved discrepancies by consensus.Results and discussions
High signal intensity foci were detected in 16 of 64 lesions (25%) of malignant tumors on T1-weighted images, whereas signal voids were detected in 41 of 64 lesions (64%) on SWAN (Fig. 1-3). Neither high signal intensity hemorrhagic foci on T1-weighted images nor signal voids due to hemorrhage on SWAN was detected in all 16 benign tumors (Fig. 4, 5). The sensitivity, specificity, NPV, and PPV for T1-weighted images were 25%, 25%, 25%, and 100%, and for SWAN were 64%, 41%, 41%, and 100%, respectively. These results may be because high signal intensity due to the T1 shortening effect of methemoglobin may reflect only subacute hemorrhage, whereas signal voids on SWAN may reflect all phases of hemorrhage, especially both deoxyhemoglobin in acute phase and hemosiderin in chronic phase 1,2, 4-7. Sehgal et al. reported that SWS (Susceptibility-weighted imaging: SWI) visualized blood products in high-grade malignant brain tumors and improved tumor characterization than T1-weighted images. Because high-grade, aggressive malignant tumors tend to have rapidly growing vasculature and multiple intra-tumoral hemorrhage may occur, detecting hemorrhagic areas within tumors could lead to improved determination of tumor status 1. Takeuchi et al. reported high signal intensity foci were detected in 40% of 10 uterine sarcomas on fat-saturated T1-weighted images, whereas signal voids were detected in all lesions (100%) on SWS (SWAN) and concluded that SWS is a sensitive MRI technique which demonstrates hemorrhage of varying chronicity in patients with uterine sarcomas and may improve the diagnostic ability 2. In the current study, intra-tumoral hemorrhage was specific for malignant ovarian tumors with high PPV (100%) and considered as an independent factor which is suggestive for malignancy. SWS (SWAN) was sensitive for intra-tumoral hemorrhage in malignant ovarian tumors compared with T1-weighted images, and considered as useful sequence in differentiating benign and malignant ovarian tumors.Conclusion
SWS is a sensitive MR technique which demonstrates intra-tumoral hemorrhage of malignant ovarian tumors, and we conclude that the demonstration of intra-tumoral hemorrhage in patients with ovarian tumors by SWS may provide valuable diagnostic findings.Acknowledgements
No acknowledgement found.References
- Sehgal V, et al. Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging 24:41-51, 2006.
- Takeuchi M, et al. Clinical utility of susceptibility-weighted MR sequence for the evaluation of uterine sarcomas. Clin Imaging. 53:143-50, 2019.
- Thomassin-Naggara I, et al. Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS MRI) Score for Risk Stratification of Sonographically Indeterminate Adnexal Masses. JAMA Netw Open. 3:e1919896, 2020.
- Haacke EM, et al. Susceptibility weighted imaging (SWI). Magn Reson Med. 52:612-8, 2004.
- Boeckh-Behrens T, et al. Susceptibility-weighted angiography (SWAN) of cerebral veins and arteries compared to TOF-MRA. Eur J Radiol 81:1238-45, 2012.
- Löbel U, et al. Three-dimensional susceptibility-weighted imaging and two-dimensional T2*-weighted gradient-echo imaging of intratumoral hemorrhages in pediatric diffuse intrinsic pontine glioma. Neuroradiology 52:1167-77, 2010.
- Takeuchi M, et al. Susceptibility-weighted MRI of extra-ovarian endometriosis: preliminary results. Abdom Imaging 40:2512-6, 2015.
Figures
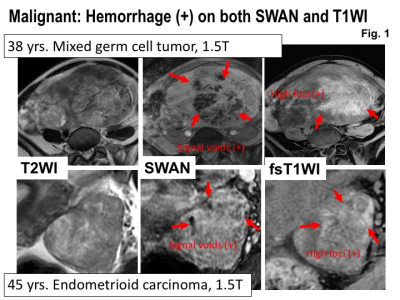
Malignant: Hemorrhage (+) on both SWAN and T1WI. Upper: MRI of 38 years woman with mixed germ cell tumor.Lower: MRI of 45 years woman with endometrioid carcinoma. Intra-tumoral hemorrhage is revealed as high signal intensity areas on fat-saturated T1WI and as signal voids on SWAN.
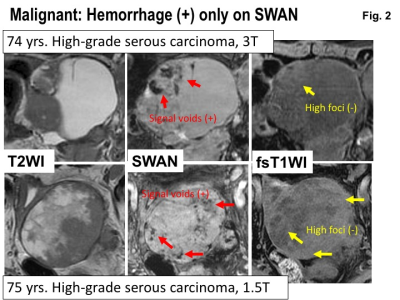
Malignant: Hemorrhage (+) only on SWAN. Upper: MRI of 74 years woman with high-grade serous carcinoma.Lower: MRI of 75 years woman with high-grade serous carcinoma. Intra-tumoral hemorrhage is revealed as signal voids on SWAN, but is not observed on fat-saturated T1WI.
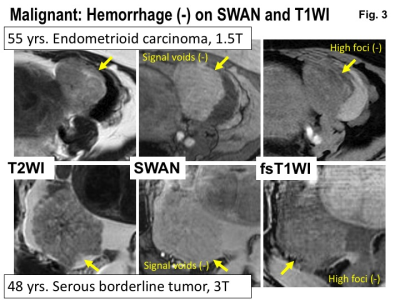
Malignant: Hemorrhage (-) on SWAN and T1WI. Upper: MRI of 55 years woman with endometrioid carcinoma.Lower: MRI of 48 years woman with serous borderline tumor. Intra-tumoral hemorrhage is not revealed both on SWAN and fat-saturated T1WI.
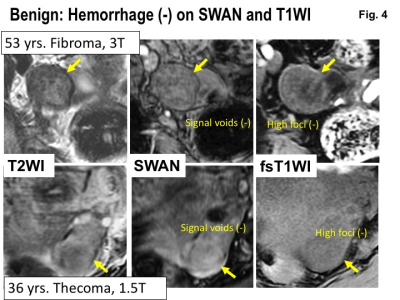
Benign: Hemorrhage (-) on SWAN and T1WI. Upper: MRI of 53 years woman with fibroma. Lower: MRI of 36 years woman with thecoma. Intra-tumoral hemorrhage is not revealed both on SWAN and fat-saturated T1WI.
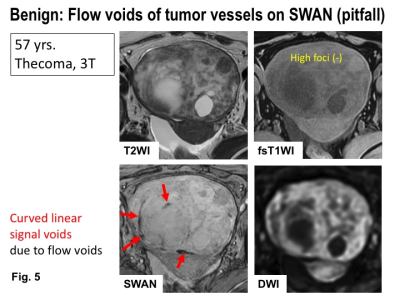
Benign: Flow voids of tumor vessels on SWAN (pitfall). MRI of 57 years woman with thecoma. Intra-tumoral hemorrhage is not revealed both on SWAN and fat-saturated T1WI. Curved linear signal voids due to flow voids of tumor vessels are observed on SWAN.
DOI: https://doi.org/10.58530/2022/3673