3658
Breast DWIBS with patient-adaptive STIR inversion delay (TI) optimization utilizing dynamic TI scout scan (DynTI)
Mana Kato1, Kazuo Kodaira1, Yasuhiro Goto1, Yasutomo Katsumata2, Mai Nishihara2, Masami Yoneyama2, Isao Shiina1, Yutaka Hamatani1, Takumi Ogawa1, Michinobu Nagao3, and Shuji Sakai3
1Radiological Services, Tokyo Woman's Medical University Hospital, Tokyo, Japan, 2Philips Japan, Tokyo, Japan, 3Diagnostic imaging & Nuclear Medicine, Tokyo Woman's Medical University Hospital, Tokyo, Japan
1Radiological Services, Tokyo Woman's Medical University Hospital, Tokyo, Japan, 2Philips Japan, Tokyo, Japan, 3Diagnostic imaging & Nuclear Medicine, Tokyo Woman's Medical University Hospital, Tokyo, Japan
Synopsis
DWI provides beneficial information that has high diagnostic potential of breast lesions. DWIBS can suppresses the background signals including fat tissues effectively, but there are often insufficient fat suppression cases because of non-adaptation TInull were used. In this study we demonstrated that the actual TI for nulling breast fat in DWI is patient-specific and dynamic TI scout scan (DynTI) is useful to explore patient-adaptive TI that can provide more beneficial to increase the robustness of breast DWIBS.
Introduction
Diffusion-weighted magnetic resonance imaging (DWI) provides clinically useful information based on the diffusivity of water molecules and could detect oncological lesions in the human body1. Breast MRI has the highest sensitivity for breast cancer detection compared with other imaging modalities, and breast DWI has shown a high diagnostic potential in detection of malignant lesions2. However, breast DWI is still challenging and often suffers from signal ununiformly and insufficient fat suppression due to both B0 and B1 inhomogeneities3,4. Diffusion-weighted imaging with background body signal suppression (DWIBS), which combines a STIR (short tau inversion recovery) pulse for fat suppression instead of spectrally selective pulse, sufficiently suppresses the background signals including fat tissues5. The optimal inversion delay for nulling fat tissues (TInull) is commonly determined either by following a literature6 (TInull=260ms) or by calculating the online tool which enables adequate TI according to the numerical simulations7 (TInull=235ms). In fact, we still encountered insufficient fat suppression cases with the DWIBS-based scans even though applying TInull values by using aforementioned methods. We hypothesized that TInull can be changed by individual breast structures and/or conditions, these may be depending on the patients. It is challenging to define such patient specific TI in clinical practice8. In this study, we investigate whether TInull can be changed by individual breast structures/conditions in each patient and propose a new visual approach for patient-adaptive TI optimization utilizing dynamic TI scout scan (DynTI), prior to breast DWIBS scans.Methods
All MR imaging was performed on a 3.0T clinical system (Ingenia, Philips Healthcare) using the dStream Breast coil with the patient in prone position. First, we used Dynamic TI scout scan (DynTI) sequence to investigate patient-specific optimal TI in respective 14 volunteers (age range: 24-85). DynTI for exploring optimal TI of the DWIBS combines with the dynamic scan procedure, but the scan parameters (including TR, TE, number of slices, averages, etc.) of each DW-EPI scan are exactly same. TI is only automatically increasing with the number of dynamic scans (TI increments: 5ms/dyn) and initial TI (original TI definition from the use interface) is unaffected. Consequently, DynTI enables a direct visualization of images with different TIs in a short scan time to allow actual clinical exams with the optimal setting. The imaging-parameters of DynTI: TR/TE= 6000/78ms, FOV= 350×278mm, voxel size= 4.4×4.48×5.0 mm, scan time= 3:30. Subsequently, we defined the patient-specific TInull in respective breast cases by measuring time intensity curves of the mean signal intensities obtained by each dynamic with different TIs. Finally, DWIBS images were acquired in the transverse plane. We compared three types of STIR TIs: 235ms (from numerical simulations), 260ms (from literature), and patient-adaptive TI defined by DynTI images. The imaging-parameters of DWIBS: b-values= 0 and 1000 s/mm2, TR/TE= 6000×78ms, FOV= 350×299mm, voxel size= 4.5×4.5×5.0mm, SENSE factor= 1.8, scan time= 2:00. For quantitative comparison, mean signal intensity was measured by placing the circular region of interest (ROI) at both right and left sides of breast fat regions on the center slice and both upper and lower edges of the slices, respectively. Wilcoxon signed-rank tests were performed to compare mean signal intensities among TI of 235ms, 260ms and patient-adaptive TIs.Results & Discussion
Figure 2 shows the representative DynTI images and time intensity curve. DynTI clearly demonstrated a direct visualization of images with different TIs with actual DWIBS scan parameter setting. The TInull values determined by DynTI in all cases are shown in Figure 3. Actual TInull values in respective cases were patient-specific as we expected, it strongly suggests that patient-adaptive TI definition prior to DWIBS scan is important to obtain robust fat suppression. Figure 4 and 5 show the comparison of signal intensities of the fat tissues (Fig. 4) and representative DWIBS images among three TI delay times (Fig. 5). Mean signal intensity of DynTI-based patient-adaptive TI delay time was significantly lower compared with that of 260ms and 235ms. The arrows indicate insufficient fat suppression on non-adaptation TI delay times (235 and 260 ms) (Fig. 5). DynTI-based patient-adaptive TI showed highest quality of fat suppression and signal homogeneity in all slices compared with other TI delay times.Conclusion
We have demonstrated that the actual inversion delay time for nulling breast fat signals in DWI is patient-specific and DynTI is useful to visually explore patient-adaptive TI that can provide more beneficial to increase the robustness of breast DWIBS.Acknowledgements
No acknowledgements found.References
(1) Thomas C. Kwee et al. Whole-body diffusion-weighted magnetic resonance imaging. European Journal of Radiology. 2009 Aug; 70: 409-417 (2) Doris Leithner, MD et al. Abbreviated MRI of the Breast: Does It Provide value? Journal of Magnetic Resonance Imaging. 2019 Jun; 49 (7): e85-e100 (3) Ashkan A. Malayeri, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. RadioGraphics. 2011 Oct; 31 (6): 1773-91 (4) Dow-Mu Koh et al. Diffusion-Weighted MRI in the Body: Applications and Challenges in Oncology. AJR Am j Roentgenol. 2007 June; 188 (6): 1622-35 (5) Taro Takahara et al. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004 Jul-Aug; 22(4): 275-82 (6) Caroline Mesmann et al. Evaluation of image quality of DWIBS versus DWI sequences in thoracic MRI at 3T. Magn Reson Imaging. 2014 Dec; 32(10): 1237-41 (7) Miho Kita et al. Online tool for calculating null points in various inversion recovery sequences. Magn Reson Imaging. 2013 Nov; 31 (9): 1631-1639 (8) Sai Man Cheung et al. Intra-tumoural lipid composition and lymphovascular invasion in breast cancer via non-invasive magnetic resonance spectroscopy. Eur Radiol. 2021 Jun;31(6):3703-3711.Figures
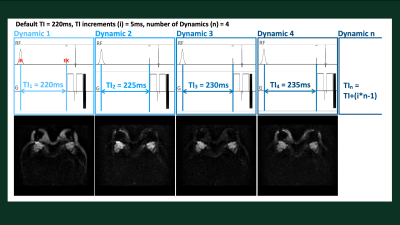
Figure 1. Scheme of dynamic TI scout scan (DynTI).
A total of 11 dynamic scans are applied, TI is automatically increasing from
220 (original TI defined from the user interface) to 270ms with the increment
of 5ms per dynamic scan.
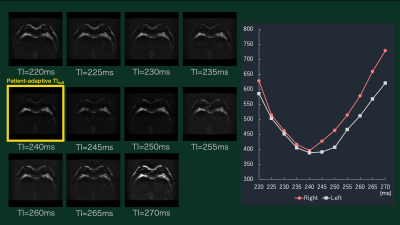
Figure 2. Representative DynTI images and thereby
time intensity curve. Each dynamic scan images show different TIs, the optimal
TI for null the breast fat can be visually assessed by the time signal
intensity curves.
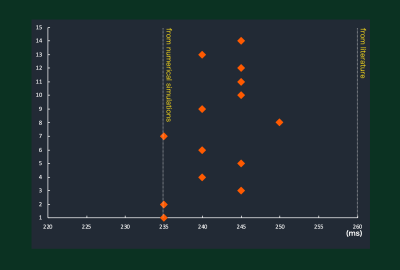
Figure 3. The TInull values determined
by DynTI images in 14 volunteers. Actual TInull values in all
subjects were varied and patient-specific.
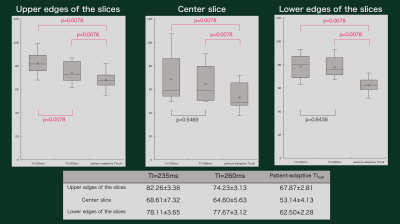
Figure 4. The results of signal intensities
of the fat tissues. Mean signal intensity of DynTI-based patient-adaptive TI
delay time was significantly lower compared with that of 260ms and 235ms in all
slices.
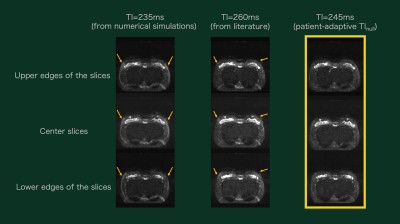
Figure 5. Representative DWIBS images. The
arrows indicate insufficient fat suppression on non-adaptation TI delay times (235
and 260 ms). DynTI-based patient-adaptive TI showed highest quality of fat suppression
and signal homogeneity in all slices compared with other TI delay times.
DOI: https://doi.org/10.58530/2022/3658