3657
Applying Multiple Diffusion Models to Predict HER-2 Status in Patients with Breast Cancer: a preliminary study using Diffusion Spectrum Imaging1Department of Radiology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China, 2MR Scientific Marketing, Siemens Healthineers, Guangzhou, China, 3MR Scientific Marketing, School of Physics and Electronic Science, Shanghai, China
Synopsis
This study explored the feasibility of applying multiple diffusion models based on the same diffusion spectrum imaging (DSI) data into preoperative prediction of HER-2 status in patients with breast cancer (BC). The results showed that some diffusion parameters can discriminate HER-2 positive BC patients, but apparent diffusion coefficient (ADC) based on conventional mono-exponential model can not. This study suggests that the simultaneously application of multiple diffusion models from DSI data is feasible for diagnosis of patients with BC, which has certain clinical value of preoperative prediction of HER-2 status and is worthy of further research.
Introduction
In clinical practice, HER-2 status in patients with breast cancer is determined by surgery or biopsy, both of which are invasive. As a noninvasive method, conventional diffusion magnetic resonance imaging (dMRI), including diffusion weighted imaging (DWI), diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI), remains inadequate in discriminating HER-2 status in BC patients. DSI is model-freely reconstructed by using multiple b-values and gradient directions in the entire q-space to sample diffusion signals of water molecules, and quantitatively estimates them by probability density function $[1]$, which is mathematically and physically advantageous over the other diffusion magnetic resonance imaging (dMRI) techniques $[2]$. More important, DSI data contains sufficient b-values and directions for accurate estimation of multiple other diffusion models, including DTI, DKI, mean apparent propagator MRI (MAP-MRI), and neurite orientation dispersion and density imaging (NODDI). In recent years, the idea of simultaneously usage of multiple diffusion models was successfully applied in diseases of central nervous system $[3]$. In this study we preliminarily explored the feasibility of this technique in preoperative prediction of HER-2 status in BC patients.Methods
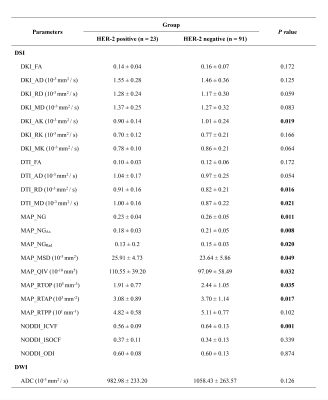
This study included 114 BC patients who underwent conventional MRI, DWI, dynamic contrast-enhanced MRI (DCE-MRI), and DSI before surgery or biopsy on a 3T MR system (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany). Conventional MRI including T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) sequences was performed to diagnose BC. ADC map was generated by DWI (b-value of 0 and 800 s/mm2). DCE-MRI was performed with an acceleration-VIBE (CAIPIRINHA-VIBE) sequence after the intravenous administration for measuring tumor size. DSI data was acquired by using a full q-space Cartesian grid sampling procedure with spectral attenuated inversion recovery (SPAIR) technique, with 9 b-values (0, 200, 450, 650, 900, 1100, 1350, 1800 and 2000 s/mm2), along with 2, 6, 12, 8, 6, 24, 24, 12 and 6 directions, respectively. Parameters were as follows: TR = 6600 ms, TE = 97 ms, slice thickness = 4 mm, slice gap = 0.8 mm, and acquisition time = 11 min 33 sec. Multiple diffusion models, including DTI, DKI, MAP-MRI, and NODDI, were reconstructed based on DSI data using the software (NeuDiLab) developed in-house based on the open-resource tool DIPY (Diffusion Imaging In Python, http://nipy.org/dipy), and the corresponding diffusion parameters were calculated simultaneously. Differences in diffusion parameters derived from DTI, DKI, NODDI, MAP models and conventional mono-exponential model were assessed between HER-2 negative (n = 91) and HER-2 positive BC patients (n = 23) by using the independent t-test. Diagnostic performances of the diffusion parameters with significant differences were determined by receiver operating characteristic (ROC) analysis. P < 0.05 was considered statistically significant.Results
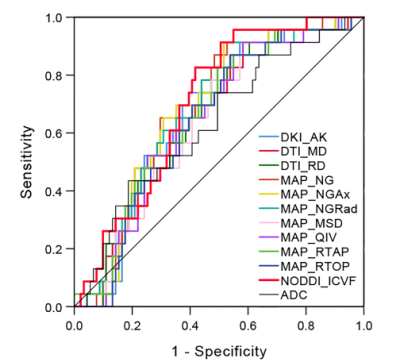
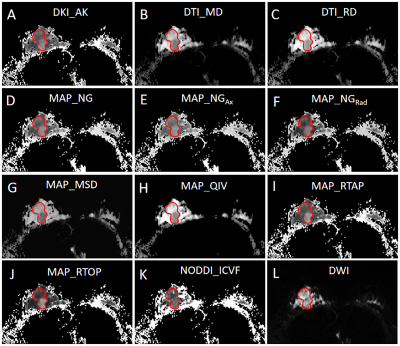
Comparisons of the quantitative parameters derived from multiple diffusion models between HER-2 positive and HER-2 negative BC patients are shown in Table 1. ADC from mono-exponential model showed no statistically significant difference between HER-2 positive and HER-2 negative BC patients (P > 0.05). Some parameters based on DTI, DKI, MAP-MRI, and NODDI models were significantly different between HER-2 positive and HER-2 negative BC patients (P value ranged from 0.001 to 0.049). According to ROC analyses, the AUCs of those significant DSI quantitative parameters ranged from 0.628 to 0.700 in distinguishing HER-2 status in BC patients (Figure 1). The diffusion parameter maps of a representative HER-2 positive BC patient are shown in Figure 2.Discussion & Conclusion
This study was a preliminary feasibility study of multiple advanced neural diffusion models from DSI data for the preoperative prediction of HER-2 status in BC patients. Generally, the results showed that many diffusion parameters from neural diffusion models outperformed the conventional mono-exponential models in discriminate HER-2 status in BC patients. More specifically, two points need considered here: 1) ICVF (NODDI), RTTP(MAP), NG(MAP) parameters showed high performance. The ICVF and RTTP parameter reflects intra-cellular fraction and probability of restricted diffusion, which indicates that the tumor cell density and intra-cellular volume fraction were related to HER-2 status; 2) performance of the direction sensitive parameters showed no significant differences, such as diffusion coefficient and kurtosis coefficients, and the FA is also rather low below 0.2, which indicates isotropic diffusion environment for BC. Thus, to conclude, successfully applied neural diffusion models in differentiated HER-2 status, and the results showed that the parameters reflecting intra-cellular and restricted diffusion fraction outperformed the other parameters. In further study, comparing to the direction related parameters, parameters reflecting more specific information of the cellular size, density or tissue anisotropy from cancer models could be applied for comparison.Acknowledgements
We thank all the volunteers who participates in this research.References
[1] Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54(6):1377-1386. doi:10.1002/mrm.20642.[2] Varela-Mattatall GE, Koch A, Stirnberg R, et al. Comparison of q-Space Reconstruction Methods for Undersampled Diffusion Spectrum Imaging Data. Magn Reson Med Sci. 2020;19(2):108-118. doi:10.2463/mrms.mp.2019-0015.[3] Mao J, Zeng W, Zhang Q, et al. Differentiation between high-grade gliomas and solitary brain metastases: a comparison of five diffusion-weighted MRI models. BMC Med Imaging. 2020;20(1):124. Published 2020 Nov 23. doi:10.1186/s12880-020-00524-w.
Figures