3655
SNR boost in whole-body DWIBS utilizing deep learning constrained Compressed SENSE reconstruction1Philips Japan, Tokyo, Japan, 2Tokyo Metropolitan Police Hospital, Tokyo, Japan, 3Philips Healthcare, Best, Netherlands, 4Philips GmbH Market DACH, Hamburg, Germany
Synopsis
Diffusion weighted whole body imaging with background body signal suppression (DWIBS) is an important tool for whole body imaging to e.g. visualize malignant tumor and abnormal lymph nodes. Next to the low intrinsic SNR of DWIBS, direct coronal DWIBS can suffer from another SNR penalty due to the high acceleration factors to minimize distortion. In this work, we compare SENSE, C-SENSE and C-SENSE-AI (CS-AI) accelerated direct coronal DWIBS scans. Results indicate that CS-AI clearly reduces the noise artifacts, boosts the overall image SNR, and improves lesion conspicuity.
PURPOSE
Diffusion weighted whole body imaging with background body signal suppression (DWIBS)1 is widely used for whole-body diffusion-weighted imaging. DWIBS can visualize malignant tumors and metastases and abnormal lymph nodes and is useful for determining the response of bone metastasis to treatment2-6. Single-shot echo-planar imaging (EPI) is a first-choice sequence for DWIBS in routine clinical examinations, but it sometimes suffers from severe image distortion due to the presence of air within and/or at the edge of the FOV, especially at the border of chest and abdomen. To solve this problem, direct coronal acquisition with higher SENSE (sensitivity encoding) acceleration factor around 4.0~5.5 is recently applied7, it can reduce the distortion efficiently. However, using such a higher acceleration factor often results in increased noise artifacts on the images due to the high geometry factor related to the coil geometry characteristics8,9.Recently, integrating artificial intelligence (AI) into the Compressed SENSE reconstruction (CS-AI), based on Adaptive-CS-Net10,11, has been introduced and CS-AI dramatically reduced noise artifacts and significantly improved the accuracy and robustness of T2 values in submillimeter high-resolution prostate T2 mapping with very high acceleration factor (R=12)12. In addition, it has been shown recently that deep learning-based denoising is an effective tool for image quality improvement in whole-body DWI images13. We hypothesize that the CS-AI reconstruction will improve the SNR of direct-coronal DWIBS. In this study, we attempt to utilize the CS-AI framework for reducing the noise artifacts in single-shot DW-EPI images. The purpose of this study is to demonstrate the feasibility of CS-AI in whole-body DWIBS with direct coronal acquisition.
METHODS
A total of five volunteers/patients were examined on a 3.0T whole-body clinical system (Ingenia Elition X, Philips Healthcare). The study was approved by the local IRB, and written informed consent was obtained from all subjects. CS-AI-DWI images were compared with conventional SENSE and CS-DWI images for image quality, especially for the reduction of image noise.CS-AI DWI is based on single-shot DW-EPI acquisition with a regular sampling pattern. The CS-AI framework is used for reconstruction. The CS-AI model used in this study is the extension of the Adaptive-CS-Net algorithm. In CS-AI, the iterative optimization procedure in the C-SENSE reconstruction chain is unrolled for a fixed number of Unet type of blocks. The model was trained on more than 700,000 images, including 2D and 3D data, and multiple contrasts and anatomical areas.
Imaging parameters for DWIBS were as follows: 3-stations, coronal acquisition, voxel size=4.5*4.5*4.5mm3, FOV=290*504mm, 66slices, b-value=0 and 1000s/mm2, TR=5439ms, TE=64ms, SENSE/C-SENSE acceleration factor = 3 or 5.5, and total acquisition time=3min15s per station.
RESULTS and DISCUSSION
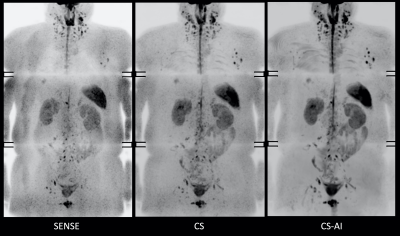
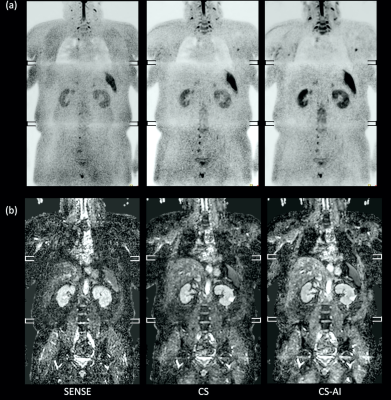
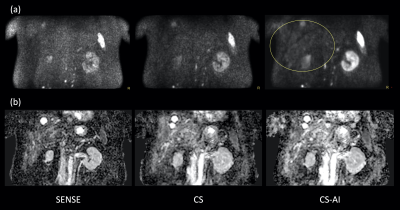
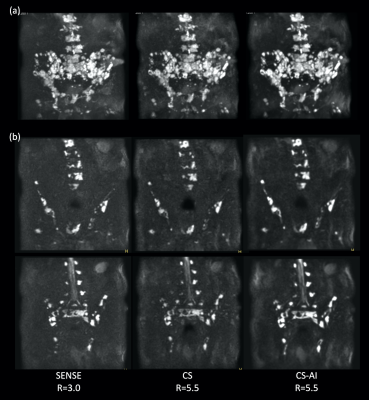
Fig.1 and 2 show representative whole-body DWIBS MIP images of a relatively skinny volunteer and an obese volunteer. Fig.3 also shows representative b=1000s/mm2 source images and corresponding ADC maps of the second volunteer. CS reduced the noise that spreads throughout the SENSE-DWI images and ADC maps, and CS-AI even further cleaned up the noise compared to CS, regardless of the volunteers’ size. Moreover, in both cases, CS-AI improved the image contrast and showed better visualization of spinal cord, spine vertebrae, peripheral nerves and lymph nodes. Fig. 4 shows representative source images and ADC maps for DWIBS with b=1000s/mm2. Liver parenchyma (yellow circle) in CS-AI DWI and ADC images were more conspicuous compared with SENSE and CS.Fig.5 shows the representative clinical images of a patient with prostate cancer and multiple bone metastases with b=1200s/mm2. In this case, we compared SENSE with acceleration factor=3 and CS/CS-AI with acceleration factor=5.5. By increasing the acceleration factor, CS and CS-AI improved the image distortion, especially for the upper spine. Also, CS-AI reduced the image noise and improved the lesion conspicuity. Although further clinical investigation is needed, CS-AI might significantly improve the robustness and lesion conspicuity of whole-body direct-coronal DWIBS regardless of the patients’ size.
CONCLUSION
Whole-body direct-coronal DWIBS with CS-AI clearly reduces the noise artifacts and boosts the overall image SNR compared to conventional SENSE- and CS-DW-EPI. Initial experience indicates that lesion conspicuity is improved as well. This technique may be helpful for whole-body cancer assessment.Acknowledgements
No acknowledgement found.References
1. TakaharaT, et al, Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. Jul-Aug 2004;22(4):275-82.
2. Fruehwald-Pallamar J et al. Functional imaging in head and neck squamous cell carcinoma: correlation of PET/CT and diffusion-weighted imaging at 3 Tesla. Eur J Nucl Med Mol Imaging. 2011 Jun;38(6):1009-19.
3. Michielsen KL et al. Whole-body diffusion-weighted magnetic resonance imaging in the diagnosis of recurrent ovarian cancer: a clinical feasibility study. Br J Radiol. 2016 Nov;89(1067):20160468.
4. Pearce T et al. Bone metastases from prostate, breast and multiple myeloma: differences in lesion conspicuity at short-tau inversion recovery and diffusion-weighted MRI. Br J Radiol. 2012 Aug;85(1016):1102-6.
5. Perez-Lopez R et al. Diffusion-weighted Imaging as a Treatment Response Biomarker for Evaluating Bone Metastases in Prostate Cancer: A Pilot Study. Radiology. 2017 Apr;283(1):168-177.
6. Sun M et al. Application value of diffusion weighted whole body imaging with background body signal suppression in monitoring the response to treatment of bone marrow involvement in lymphoma. J Magn Reson Imaging. 2016 Dec;44(6):1522-1529.
7. Suzuki M, et al. Artifact-robust diffusion-weighted whole-body imaging with background suppression at 3 T using improved turbo spin-echo diffusion-weighted imaging. Br J Radiol. 2019 Feb;92(1094):20180489. doi: 10.1259/bjr.20180489.
8. Patricia N, et al. Parallel Imaging Artifacts in Body Magnetic Resonance Imaging. Can Assoc Radiol J. 2009;60: 91–98.
9. Yanasak NE, et al. MR imaging artifacts and parallel imaging techniques with calibration scanning: a new twist on old problems. Radiographics. 2014;34:532-48.
10. Pezzotti N, et al. An Adaptive Intelligence Algorithm for Undersampled Knee MRI Reconstruction. IEEE Access. 2020;8:204825-204838.
11. Pezzotti N, et al. Adaptive-CS-Net: FastMRI with Adaptive Intelligence. arxiv. 2019;(NeurIPS).
12. Yoneyama M, et al. Rapid submillimeter high-resolution prostate T2 mapping with a deep learning constrained Compressed SENSE reconstruction. Proc Intl Soc Mag Reson Med. 2021:4117.
13. Zormpas-Petridis K, et al. Accelerating Whole-Body Diffusion-weighted MRI with Deep Learning-based Denoising Image Filters. Radiol Artif Intell. 2021 Jul 14;3(5):e200279. doi: 10.1148/ryai.2021200279.
Figures
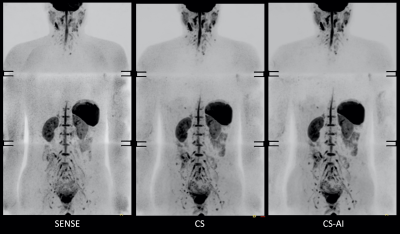
Figure 1. Representative DWIBS MIP images using SENSE, CS and CS-AI (with acceleration factor = 5.5) of a relatively skinny volunteer.
It should be noted that in this case the spinal cord around the lung is badly visualized, probably due to unwanted water suppression using the slice-selective gradient reversal (SSGR) technique.