3647
Comparative analysis of diffusion-weighted image analysis along the perivascular space (DWI-ALPS) for evaluating interstitial fluid status1Department of Innovative Biomedical Visualization (iBMV), Nagoya University, Nagoya, Japan, 2Department of Radiology, Nagoya University, Nagoya, Japan, 3Devision of Radiology, Nagoya University Hospital, Nagoya, Japan, 4Canon Medical Systems Corporation, Otawara, Japan
Synopsis
The diffusion tensor image analysis along the perivascular space (DTI-ALPS) method has been developed to evaluate glymphatic function. In order to evaluate wider clinical cases, we developed a diffusion weighted image ALPS (DWI-ALPS) technique. The ALPS-index was retrospectively calculated by clinical DWI of normal subjects and those with pathologies. In normal subjects, the ALPS-index was highest in those in their forties and lowest in those in their seventies. Patients with Alzheimer’s disease and subdural hematoma showed a lower ALPS-index than age-matched normal subjects. The DWI-ALPS method seems to be feasible for evaluation of glymphatic function in the clinical practice.
Purpose
The glymphatic system is a system for waste drainage via the cerebrospinal fluid or interstitial fluid along the perivascular space of the brain. In animal experiments, the activity of the glymphatic system has been evaluated by intrathecal administration of tracers (1). Several studies have evaluated glymphatic function in human subjects by intrathecal administration of gadolinium-based contrast agent tracers (2). As a non-invasive method to evaluate glymphatic function by using diffusion tensor imaging (DTI), the “diffusion tensor image analysis along the perivascular space (DTI-ALPS)” technique has been developed (3). In this method, the analysis along perivascular space (ALPS) index, which is the ratio of water diffusivity along the perivascular space, is used to indicate glymphatic function, and many publications have demonstrated glymphatic insufficiency in various pathologies by using this method (4-7). In the current study, we developed a method to use diffusion-weighted imaging (DWI) with a three-axis motion-proving gradient to obtain the DWI-ALPS index. We evaluated the correlation between age and ALPS index in normal subjects and evaluated the difference in the DWI-ALPS index of various pathologies.Subjects and Methods
This retrospective study undergone with permission from the institutional review board. The study included the data of 118 cases who underwent magnetic resonance imaging (MRI) studies including DWI and were found to have no abnormal findings in the brain. We also examined DWI of the cases with pathologies, including Alzheimer’s disease (17 cases), Parkinson’s disease (11 cases), subdural hemorrhage (16 cases), and moyamoya disease (14 cases). The DWI included in the clinical sequence was as follows: echo-planar imaging; repetition time, 5000 ms; echo time, 85 ms; motion-proving gradient, orthogonal 3 axes; b-value, 1000 s/mm²; number of averages, 1 for b=0 and 2 for b=1000; acquisition time, 1 min 11 s; axial imaging plane on the anterior commissure-posterior commissure (AC-PC) line.We retrospectively generated apparent diffusion coefficient (ADC) images in the x-, y-, and z-axes and created composite color images in the same manner with color fractional anisotropy images of DTI. On the slice including the body of the lateral ventricle of the composite color image, we identified the projection fiber and association fiber areas and measured ADC values along the x-, y-, and z-axes to calculate the DWI-ALPS index, which is given by the ratio of the mean of the x-axis ADC in the area of the projection fiber (ADCxproj) and the x-axis diffusivity in the area of association fibers (ADCxassoc) to the mean of the y-axis diffusivity in the area of projection fiber (ADCyproj) and z-axis diffusivity in the area of association fibers (ADCzaccoc) as follows:
DWI-ALPS index=mean (ADCxproj, ADCxassoc)/mean (ADCyproj, ADCzassoc)
The value of the DWI-ALPS index in normal subjects was examined with age, and comparisons by age group were made by analysis of variance (ANOVA) test. We also performed a polynomial regression between the DWI-ALPS index and age. For patients with Alzheimer’s disease, Parkinson’s disease, subdural hemorrhage, and moyamoya disease, we compared the DWI-ALPS index between the disease group and age-matched normal subjects.
Results
Plots of the DWI-ALPS index according to the age of normal subjects are shown in Figure 1a. The DWI-ALPS index was higher in patients in their forties compared to other age groups and lower in those in their seventies (Figure 1b). Using polynomial regression, there was a statistically significant correlation (p<0.05) in secondary regression, as shown by the blue dotted line in Figure 1a.Compared with age-matched normal subjects, patients with Alzheimer’s disease (Figure 2) showed a statistically significant difference (p<0.05) in the DWI-ALPS index, those with Parkinson’s disease did not show a statistically significant difference (Figure 3), and those with subdural hemorrhage (Figure 4) showed a significantly (p<0.05) lower DWI-ALPS index. The DWI-ALPS index plots of patients with moyamoya disease overlapped with those of normal subjects, and there was no statistically significant difference (Figure 5).
Discussion
In the current study, DWIs acquired in clinical MRI examinations were utilized to calculate the DWI-ALPS index in patients with a wide age range. In the polynomial regression, only secondary regression, and not the linear regression, showed a significant correlation. The regression line showed a peak for patients in their thirties and forties, which was supported by the result of the ANOVA. This may suggest that glymphatic function is most efficient in the 30 –40 age group compared to older or younger age groups.The DWI-ALPS index-to-age plot seems to be useful in the evaluation of the DWI-ALPS index in pathological states. In the current study, both the Alzheimer’s and Parkinson’s disease groups showed lower mean DWI-ALPS indexes; however, only the Alzheimer’s disease group showed a significant difference compared to normal subjects. Interestingly, the subdural hemorrhage group also showed a significantly lower DWI-ALPS index, probably because glymphatic function is impaired in patients with subdural hemorrhage.
Conclusion
The DWI-ALPS index calculated from commonly employed DWI showed a higher value in the 30–40 age group. The DWI-ALPS index could also delineate glymphatic dysfunction in patients with Alzheimer’s disease and subdural hematoma. While DWI requires a shorter imaging time and is widely used in clinical practice, the DWI-ALPS method seems to be useful to evaluate glymphatic function in various clinical studies.Acknowledgements
No acknowledgement found.References
1. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012;4(147):147ra111.
2. Ringstad G, Valnes LM, Dale AM, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018;3(13).
3. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol 2017;35(4):172-178.
4. Yokota H, Vijayasarathi A, Cekic M, et al. Diagnostic Performance of Glymphatic System Evaluation Using Diffusion Tensor Imaging in Idiopathic Normal Pressure Hydrocephalus and Mimickers. Curr Gerontol Geriatr Res 2019;2019:5675014.
5. Steward CE, Venkatraman VK, Lui E, et al. Assessment of the DTI-ALPS Parameter Along the Perivascular Space in Older Adults at Risk of Dementia. J Neuroimaging 2021;31(3):569-578.
6. McKnight CD, Trujillo P, Lopez AM, et al. Diffusion along perivascular spaces reveals evidence supportive of glymphatic function impairment in Parkinson disease. Parkinsonism Relat Disord 2021;89:98-104.
7. Zhang W, Zhou Y, Wang J, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage 2021;238:118257.
Figures
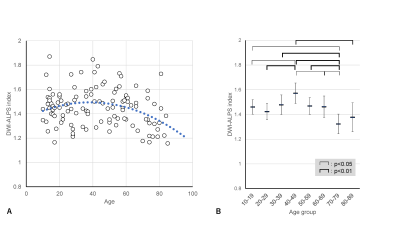
Figure 1. DWI-ALPS index-to-age plot in normal subjects.
(A) The DWI-ALPS index-to-age plot. The blue dotted line shows the secondary regression line. The DWI-ALPS index shows a peak in the 30–39 and 40-49 age groups.
(B) The distribution of age groups with means and 95% confidence intervals. The ANOVA test showed a higher index in the age group of 40–49 and lower index in the age group of 70–79.
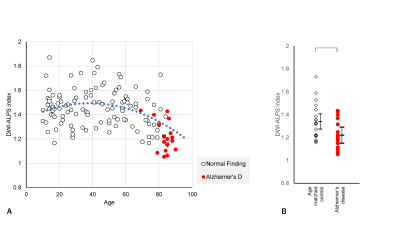
Figure 2. DWI-ALPS index-to-age plot in Alzheimer’s disease.
(A) The DWI-ALPS indexes in patients with Alzheimer’s disease are plotted in red, indicating a lower value for their age as compared to normal patients.
(B) A statistically significant difference was observed in the DWI-ALPS indexes of patients with Alzheimer’s disease compared to age-matched normal subjects.
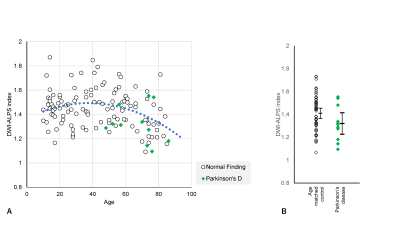
Figure 3. DWI-ALPS index-to-age plot in Parkinson’s disease.
(A) The DWI-ALPS indexes in patients with Parkinson’s disease cases are plotted in green, showing slightly lower values for their age.
(B) No statistically significant difference was observed compared to age-matched normal subjects.
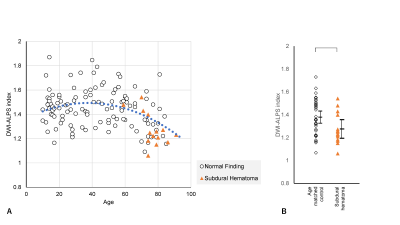
Figure 4. DWI-ALPS index-to-age plot in subdural hematoma.
(A) The DWI-ALPS indexes in patients with subdural hematoma are plotted in orange, showing a lower value for their age.
(B) A statistically significant difference was observed compared to age-matched normal subjects.
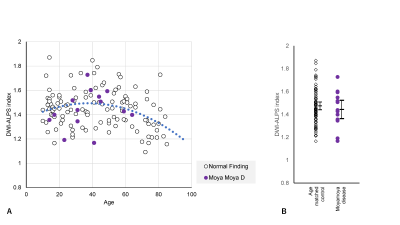
Figure 5. DWI-ALPS index-to-age plot in moyamoya disease.
(A) The DWI-ALPS indexes in patients with moyamoya disease are plotted in purple. The plots seem to overlap with those of normal subjects.
(B) No statistically significant difference was observed compared to age-matched normal subjects.