3645
Decreased brain GABA levels in patients with migraine without aura: an exploratory 1H-MRS study1Department of Radiology, The First Affiliated Hospital of Soochow University, Suzhou, China, 2Philips Healthcare, Shanghai, China, 3Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, China
Synopsis
Increasing neurophysiological studies had revealed that regional excitation-inhibition imbalance in brain played a key role in the pathogenesis of migraine. This study explored the alterations in GABA and Glx levels in the anterior cingulate gyrus (ACC) and medial prefrontal lobe (mPFC) of patients with migraine without aura (MWoA) using MEGA-PRESS at 3 Tesla. Compared with healthy controls, the GABA+ levels in the ACC and mPFC of patients were lower and correlated with attack frequency, and Glx levels had no difference, suggesting a relationship of the abnormalities of GABAergic system in the ACC and mPFC with the pathophysiological mechanism of MWoA.
Introduction
Although migraine without aura (MWoA) has a high prevalence and disability1, the effective management strategies for it are limited at present, due in part to inadequate understanding of the pathophysiological mechanism underlying this disorder. Recent neurophysiological studies emphasized the role of excitation-inhibition imbalance of the central nervous system in migraine, providing evidence for the view that the changes in neurometabolites levels within pain pathways might be responsible for migraine attack and development2, 3. The changed levels of Gamma-aminobutyric acid (GABA) and glutamate, the primary inhibitory and excitatory neurotransmitter in adult human brain, could lead to the imbalance between excitatory and inhibitory signals, thereby affecting the transmission effect of various pain signals in the ascending and descending pathways. Neuroimaging approaches revealed that MWoA patients usually had abnormal brain activities in the ACC and mPFC4-6. However, the alterations in GABA and Glx levels in the above-mentioned two regions of MWoA patients are currently not known. In this study, the GABA and Glx levels in ACC and mPFC of MWoA patients were quantitatively evaluated by MEGA-PRESS sequence, and their correlations with clinical data were analyzed.Methods
28 patients (22 female; age range 24–63 years) and 28 age-, sex-, and education level-matched healthy controls (HCs) underwent single-voxel 1H-MRS scanning at 3.0 Tesla scanner (Ingenia, Philips Healthcare, Best, The Netherlands) with a fifteen-channel phased-array head coil. The clinical indicators (disease duration, pain intensity, attack frequency and attack duration) and anxiety-depression states (GAD-7 and PHQ-9 scores) of all participants were assessed. Single volume spectra from the ACC and mPFC with the sizes of 4 × 2 × 3 cm³ and 2 × 3 × 3 cm³ were acquired using the MEGA-PRESS sequence (Fig. 1) (TR/TE = 2000/68 ms; 320 averages; number of points = 2048; spectral bandwidth = 2000 Hz; editing pulse at 1.9 ppm; editing pulse duration = 15 ms; VAPOR water suppression; scanning time = 10 min and 56 s). The GABA signal at 3.02 ppm contained the overlapping signals of macromolecules and homocarnosine7. Hence, the signals acquired in this experiment were GABA+ signals. “Gannet 3.0” software applied the single Gaussian model to fit edited GABA+ at 3.02 ppm and double Gaussian model for Glx signal at 3.75 ppm (Fig. 1). The Cr peak and water peak were both applied as reference signals to ensure the reliability and repeatability of metabolite data. In this study, spectra with full-width half-maximum (FWHM) of reference signals ≤ 12 Hz and fit errors of GABA+/Glx ≤ 15% were accepted for statistical analyses. The GABA+ and Glx levels between two groups (Independent-sample t-test) and correlation analysis (Spearman) between neurotransmitter levels and clinical characteristics of patients were analyzed by SPSS 22.0.Results
The 1H-MRS was performed on the ACC in all participants and mPFC in 21 participants in each group. The spectral data were successfully included for statistical analysis from the ACC in 27 patients and 27 HCs and mPFC in 18 patients and 18 HCs. Figure 1 showed spectroscopic VOIs location and spectra from in all subjects. Table 1 and Figure 2 showed that MWoA patients had lower GABA+ levels (Cr and Water referencing) in the ACC and mPFC compared with the HCs, with no significant difference in Glx levels (Cr and Water referencing). Figure 3 showed the negative correlation between GABA+ levels and attack frequency of MWoA patients.Discussion
This study showed that MWoA patients had lower GABA+ levels in the ACC and mPFC than in healthy volunteers, with no change in Glx levels. This was the initial study that specifically investigated the GABA levels in the ACC and mPFC of MWoA patients using MEGA-PRESS sequence. The ACC is considered to play an important role in pain modulation as well as in attention and anticipation of pain8, 9. As a relay hub, it transmits input signals after evaluating requirements from the nociceptive system, such as the trigeminovascular system, to guide adaptive behaviors. The abnormalities of GABA+ levels suggested that defects in the descending modulatory circuits of pain might be responsible for the headache attack in MWoA. The mPFC also plays a key role in the nociceptive system, encoding the cognitive component of pain, and is involved in modulating pain10, 11. The decreased GABA+ level in the mPFC may bring about impaired cognition and emotional processing of pain, which is contrary to the production of anti-nociception and coping with pain. We speculated that the injured or lost GABAergic neurons or the decline in GAD expression might contribute to the decrease in the GABA+ level, leading to the reducing inhibition in response to pain stimuli12. It facilitated the amplification of nociceptive input signals within the CNS and related pain hypersensitivity13. We further revealed the negative correlations between GABA+ levels and migraine attack frequency in the patient group. These findings reflected that the GABAergic system abnormalities in the ACC and mPFC might be an underlying neurobiological mechanism in MWoA.Conclusion
The GABA+ levels in the ACC and mPFC of MWoA patients decreased and negatively correlated with headache attack frequency, indicating that the damage, loss, or dysfunction of GABAergic system in the ACC and mPFC might be related to the neurobiological mechanism of MWoA.Acknowledgements
This work was mainly supported by National Natural Science Foundation of China (grant number 81671743); the program for Advanced Talents within Six Industries of Jiangsu province (WSW-057); and the High-level Health Personnel “six-one” Project of Jiangsu province in China (LGY2016035).
References
1. Stewart WF, Linet MS, Celentano DD, et al. Age- and sex-specific incidence rates of migraine with and without visual aura. American journal of epidemiology. 1991;134(10):1111-1120.
2. Nguyen BN, McKendrick AM, Vingrys AJ. Abnormal inhibition-excitation imbalance in migraine. Cephalalgia : an international journal of headache. 2016;36(1):5-14.
3. Vecchia D, Pietrobon D. Migraine: a disorder of brain excitatory-inhibitory balance? Trends in neurosciences. 2012;35(8):507-520.
4. Xue T, Yuan K, Cheng P, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR in biomedicine. 2013;26(9):1051-1058.
5. Yu D, Yuan K, Zhao L, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR in biomedicine. 2012;25(5):806-812.
6. Zhang Y, Chen H, Zeng M, et al. Abnormal Whole Brain Functional Connectivity Pattern Homogeneity and Couplings in Migraine Without Aura. Frontiers in human neuroscience. 2020;14:619839.
7. Shungu DC, Mao X, Gonzales R, et al. Brain γ-aminobutyric acid (GABA) detection in vivo with the J-editing (1) H MRS technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test-retest reliability. NMR in biomedicine. 2016;29(7):932-942.
8. Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neuroscience and biobehavioral reviews. 2013;37(3):340-348.
9. Navratilova E, Xie JY, Meske D, et al. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(18):7264-7271.
10. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature reviews Neuroscience. 2006;7(4):268-277.
11. Ong WY, Stohler CS, Herr DR. Role of the Prefrontal Cortex in Pain Processing. Molecular neurobiology. 2019;56(2):1137-1166.
12. Roth FC, Draguhn A. GABA metabolism and transport: effects on synaptic efficacy. Neural plasticity. 2012;2012:805830.13. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2-s15.
13. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2-s15.
Figures
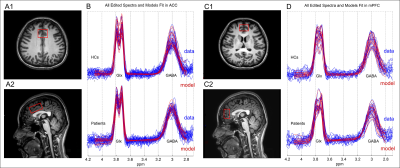
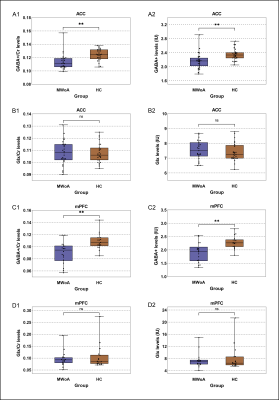
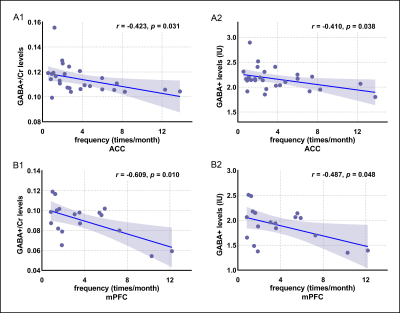
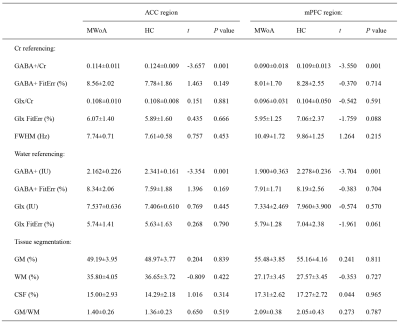
Table 1 Metabolites levels and tissue compositions of MWoA and HC groups. Continuous variables are given as mean ± standard deviation; MWoA, migraine without aura; HC, healthy control; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; IU, institutional units; FWHM, full-width at half-maximum; FitErr, fitting error; GM, gray matter; WM, white matter; CSF, cerebrospinal fluid.