3642
Distinct brain structures prospectively predict posttraumatic stress symptoms and posttraumatic growth related to COVID-191Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China
Synopsis
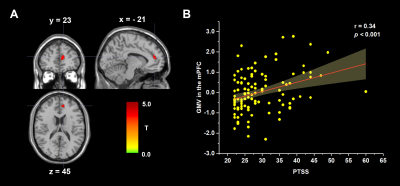
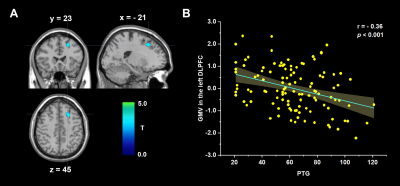
Post-traumatic stress symptoms (PTSS) and post-traumatic growth (PTG) might develop after a major trauma, but their neurological bases are largely unknown. Here, we used structural magnetic resonance imaging acquired prepandemic to explore the neuroanatomical correlates in 115 college students. The PTSS and PTG scores were collected during the epidemic. We found that PTSS was positively associated with gray matter volume (GMV) in the medial prefrontal cortex, and PTG was negatively correlated with the GMV in the dorsolateral prefrontal cortex. Our research revealed the neural underpinnings of post-traumatic consequences in healthy students, suggesting PTSS and PTG are two distinct psychological changes.
Summary of Main Findings
Using structural magnetic resonance imaging in 115 college students, we found PTSS was positively associated with gray matter volume in the medial prefrontal cortex, and PTG was negatively correlated with gray matter volume in the dorsolateral prefrontal cortex.Introduction
COVID-19 pandemic, as a worldwide health threat, could have a substantial impact on public health, including mental health 1. It is well known that, health emergencies such as epidemics can lead to harmful and long-term psychosocial consequences not only in survivors 2. In the general population, increasing number of studies have reported epidemics are related to a wide range of mental illnesses, including fear, avoidance, hyperarousal and anxiety, being prone to PTSS 3. In addition, a traumatic experience can conversely have a positive effect on peoples—known as PTG 2, which is the positive change that occurs as a result of the struggle with highly challenging life crises, such as new possibilities, relating to others, personal strength, spiritual change, and appreciation of life. A recent study showed that brain structural volume in bilateral hippocampus and amygdala were significantly larger in COVID-19 survivors compared with controls 4. However, far fewer have examined the association between PTSS and PTG and brain morphology in the general population.Therefore, the aim of the present study was to use voxel-based morphometry to examine the neuroanatomical correlates with the level of post-traumatic consequences in healthy college students after the epidemic outbreak.
Methods
A total of 115 college students (66 females, mean age =22.37, standard deviation = 2.08) who had no history of psychiatric or neurological diseases participated in this study. The Impact of Event Scale Revised scale (IES-R) 5 and post-traumatic growth inventory (PTGI) 6 were respectively employed to evaluate individuals' levels of PTSS and PTG after the epidemic outbreak. The MRI examinations were performed with a 3-Telsa Siemens MRI system using a 12-channel phase array head coil before the pandemic. We used a magnetization-prepared rapid gradient echo sequence to obtain the T1-weighted anatomical images: TR/TI/TE, 1900/900/2.26 ms; 176 slices; slice thickness, 1 mm; flip angle, 9°; matrix size, 256 × 256; voxel size, 1 mm × 1 mm × 1 mm. The MRI data was preprocessed using SPM12 software, which mainly included registration, normalization, modulation and smoothness analyses 7. To identify the brain regions, we performed whole-brain regression analyses between voxel-wise gray matter volume (GMV) and PTSS and PTG, with age, gender and total GMV as covariates. For multiple comparisons, we set the threshold for significant regions to p < 0.05 at the cluster level with an underlying p < 0.005 at the voxel level (Gaussian random field correction) 8.Results
After controlling for total intracranial volume, age and sex, whole-brain regression analyses showed that PTSS was positively associated with regional GMV in the medial prefrontal cortex (mPFC, from medial frontal cortex extending to dorsal anterior cingulate cortex; MNI coordinates: -10, 45, 18; cluster size = 203 voxels; t = 3.75; Figure 1), and PTG was negatively correlated with the regional GMV in the left dorsolateral prefrontal cortex (DLPFC, from middle frontal gyrus extending to superior frontal gyrus; MNI coordinates: -21, 23, 45; cluster size = 248 voxels; t = 4.31; Figure 2). Critically, our results persisted even after controlling for the influences of each other's level.Discussion
The current study investigated the association between PTSS and PTG and brain structure in a sample of college students during the epidemic. The finding that PTSS was positively related to GMV of the mPFC was consistent with previous meta-analysis reporting a larger mPFC volume in patients with post-traumatic stress disorder 9. The increased cortical GMVs in individuals with higher levels of PTSS might reflect a compensatory/responsive structural alteration to overcome immaturity or defects in psychosocial functions resilient to negative reaction, such as anxiety, depression and avoidance. In addition, DLPFC was proved to be the main neural correlate of PTG earlier 10, which plays a key role in adaptive growth and behavioral adjustments.Conclusion
In conclusion, this research shed light on the neural underpinnings of post-traumatic consequences in healthy students after the epidemic outbreak, and suggesting PTSS and PTG are two distinct psychological changes, providing a possible predict model to distinguish psychological reactions in college students during the COVID-19 epidemic.Acknowledgements
This work was supported by the National Natural Science Foundation (Grant Nos. 81621003, 81820108018 and 82001800), the Functional and Molecular Imaging Key Laboratory of Sichuan Province (Grant No. 2019JDS0044), the China Postdoctoral Science Foundation (Grant No. 2020M683317), and the Post-Doctor Research Project, West China Hospital, Sichuan University (Grant No. 2019HXBH104).References
1. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med Feb 20 2020;382(8):727-733.
2. Olson K, Shanafelt T, Southwick S. Pandemic-Driven Posttraumatic Growth for Organizations and Individuals. Jama Nov 10 2020;324(18):1829-1830.
3. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun Oct 2020;89:531-542.
4. Tu Y, Zhang Y, Li Y, et al. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol Psychiatry Jul 20 2021:1-6. 5. Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. Br J Psychiatry Mar 2002;180:205-209.
6. Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress Jul 1996;9(3):455-471.
7. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage Oct 15 2007;38(1):95-113.
8. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage Jun 2000;11(6 Pt 1):805-821.
9. Zhang X, Zhang J, Wang L, et al. Altered Gray Matter Volume and Its Correlation With PTSD Severity in Chinese Earthquake Survivors. Front Psychiatry 2018;9:629.
10. Nakagawa S, Sugiura M, Sekiguchi A, et al. Effects of post-traumatic growth on the dorsolateral prefrontal cortex after a disaster. Sci Rep Sep 27 2016;6:34364.
Figures