3638
Iron deposition decreased in the thalamus and functional connectivity increased between the thalamus and the hippocampus in T2DM patient1Tangdu Hospital, XI an, China, 2Shaanxi University of Chinese Medicine, Xian yang, China, 3MR Research, GE Healthcare, Beijing, China
Synopsis
In this study, change of susceptibility level in the gray matter nucleus of T2DM patients and their functional connectivity were investigated using quantitative susceptibility mapping (QSM) and fMRI techniques. We found iron deposition decreased in the thalamus and functional connectivity increased between the thalamus and the hippocampus. Susceptibility reduction suggested an involvement of chronic microglia activation in the depletion of iron from oligodendrocytes in this central and integrative brain region. The functional connectivity may indicate a compensatory enhancement. These results remind that the thalamus may play an important role in patients with T2DM.
Introduction
Iron accumulation in the brain is thought to be a pathological mechanism in patients with type 2 diabetes mellitus (T2DM).[1] Compared with other gradient echo -based imaging methods for iron quantification, such as R2* and phase methods, quantitative susceptibility mapping (QSM) offers several advantages, including higher accuracy and sensitivity in iron concentration estimation, less blooming artifacts inherent in R2* and phase approaches, and capability to distinguish paramagnetic iron versus diamagnetic calcification. This research aims to explore the difference of brain iron deposition in the gray matter nucleus between T2DM patients and healthy elderly individuals by QSM analysis and investigate whether this iron deposition affects its functional connectivity.Methods
Our Institutional Review Board approved the protocol and written informed consent was obtained from each subject. This study included 27 confirmed T2DM patients and 30 matched healthy controls. All participants underwent brain magnetic resonance examination on a 3.0T scanner (Discovery MR750, GE Healthcare) with 8-channel head coil, and 55 individuals underwent cognitive function assessments. Volumetric T1-weighted (three-dimensional brain volume imaging, 3D-BRAVO) (echo time (TE)/ repetition time (TR) = 3.2/8.2 ms, inversion time (TI) = 450 ms, flip angle (FA)= 12º, acquisition matrix = 256 × 256, slice thickness = 1 mm, slice number = 188) images were collected for anatomic structure. Brain QSM maps were computed from a 3D multiecho gradient echo (ME-GRE) data using morphology-enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference algorithm (MEDI+0). Regions of interest (ROI) were manually drawn on every slice of the caudate, putamen, pallidum. thalamus, red nucleus, substantia nigra and dentate. ITK-SNAP was used to measure the susceptibility values reflecting the content of iron in the ROIs. We compared the susceptibility values of ROIs among groups. Resting-state fMRI(rs-fMRI) included gradient-echo planar sequence sensitive to BOLD contrast (TR = 2,000 ms, TE = 30 ms, FA = 90°) and rs-fMRI was preprocessed in Data Processing Assistant for Resting-State fMRI (DPABI, http://www.restfmri.net/forum/DPARSF)[2] and the SPM12 software package in MATLAB R2013b platform. A voxel-based analysis(VBA) function connection with the differential ROIs as the seed points was further evaluated. Results were corrected for multiple comparisons using voxel-based FDR (p < 0.001) which was implemented in SPM on the MATLAB R2013b platform.Results
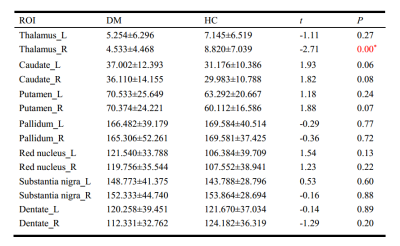
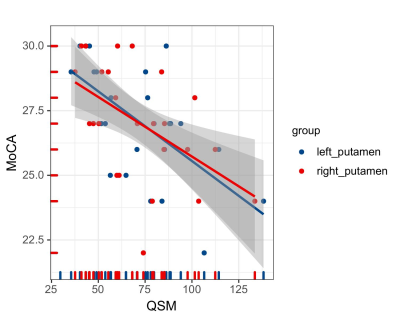
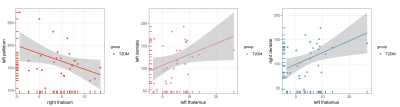
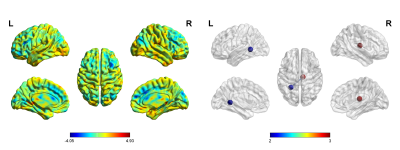
The study included 27 T2DM patients (19 males and 8 females; mean age of 52.31 ± 7.05 years) and 30 HCs (16 males and 14 females; mean age of 49.03 ± 8.06 years). These participants had no significant difference in age or gender (P > 0.05). 27 patients with T2DM and 28 HCs (14 males and 14 females; mean age, 48.96 ± 8.20 years) received an assessment of cognitive function. Two groups had no significant difference in Montreal Cognitive Assessment (MoCA) score (T2DM,27.04 ± 2.21;HCs, 26.29± 2.66). The mean susceptibility values in the right thalamus appeared obviously lower in T2DM patients than in HCs (t =2.710, P < 0.001). The susceptibility values of putamen showed obvious association with cognitive assessment scores in T2DM patients (left, r = -0.616, P = 0.001;right, r = -0.514, p = 0.009). However, an obvious correlation was observed between the changes in the susceptibility values in the pallidum and the thalamus/dentate nucleus (r = 0:400, P < 0.001; r = 0.467, P < 0.001). Futher more, T2DM patients have stronger functional connectivity between right thalamus and left hippocampus.Discussion
In this study, we found that T2DM patients’ brain susceptibility is significantly lower in thalamus, indicating decreased iron deposition. Meanwhile, functional connectivity between the thalamus and the hippocampus was found to be stronger in T2DM patients compared with HCs. The decrease in tissue iron concentration suggests an involvement of chronic microglia activation in the depletion of iron from oligodendrocytes in this central and integrative brain region. Gray matter nucleus iron is mainly derived from oligodendrocytes.[3] T2DM is characterized by chronic inflammation.[4] Inflammation leads to activation of microglia in the thalamus and release of pro-inflammatory cytokines from microglia[5], leading to release of iron from oligodendrocytes, which results in decreased magnetic susceptibility. The thalamus is the relay station for the frontal-hippocampal circuit which plays a role of memory and executive functions[6]. The stronger thalamic and hippocampal functional connectivity at this stage of T2DM may be compensatory enhancement to maintain normal executive function. Also, we found that the susceptibility values of putamen was in association with MoCA scores and the changes in the susceptibility values in the pallidum and the thalamus/dentate nucleus were associated. The striatum (include putamen) has multiple brain functions, including motor control and learning, language, reward, cognitive function, and addiction through functional cortico-striato-thalamic cortical circuits. The dentate nucleus is connected to the thalamus using the dentate thalamic tract.[7]Conclusion
Our results showed decreased iron deposition in the thalamus and enhanced functional connectivity between the thalamus and the hippocampus, indicating that thalamus may play an important role in patients with T2DM. Also, QSM might be able to help probe micro neuronal damage in gray matter and provide information on diabetic encephalopathy.Acknowledgements
No acknowledgement found.References
[1] Li J, Zhang Q, Zhang N, et al. Increased Brain Iron Deposition in the Putamen in Patients with Type 2 Diabetes Mellitus Detected by Quantitative Susceptibility Mapping. [J]. J Diabetes Res.2020;2020:7242530. DOI:10.1155/2020/7242530
[2]Yan CG, Wang XD, Zuo XN, et al. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. [J]. Neuroinformatics.2016;14(3):339-351. DOI:10.1007/s12021-016-9299-4
[3]Schweser F, Raffaini Duarte Martins AL, Hagemeier J, et al. Mapping of thalamic magnetic susceptibility in multiple sclerosis indicates decreasing iron with disease duration: A proposed mechanistic relationship between inflammation and oligodendrocyte vitality. [J]. Neuroimage.2018;167:438-452. DOI:10.1016/j.neuroimage.2017.10.063
[4]Wang Y, Wang S, Xin Y, et al. Hydrogen sulfide alleviates the anxiety-like and depressive-like behaviors of type 1 diabetic mice via inhibiting inflammation and ferroptosis. [J]. Life Sci.2021;278:119551. DOI:10.1016/j.lfs.2021.119551
[5]Toth CC, Jedrzejewski NM, Ellis CL, et al. Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. [J]. Mol Pain.2010;6:16. DOI:10.1186/1744-8069-6-16
[6]Child ND, Benarroch EE. Anterior nucleus of the thalamus: functional organization and clinical implications. [J]. Neurology.2013;81(21):1869-1876. DOI:10.1212/01.wnl.0000436078.95856.56
[7] Li J, Zhang Q, Zhang N, et al. Increased Brain Iron Detection by Voxel-Based Quantitative Susceptibility Mapping in Type 2 Diabetes Mellitus Patients With an Executive Function Decline. [J]. Front Neurosci.2020;14:606182. DOI:10.3389/fnins.2020.606182