3637
Regional Hippocampal Changes in Patients with Hyperthyroidism1NMR Research Centre, Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi, India, 2The University of Trans-disciplinary Health Sciences and Technology, Bangalore, India, 3Thyroid Research Centre, Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi, India
Synopsis
Hyperthyroid patients showed structural and functional abnormalities in the hippocampus region of the brain as suggested by neuroimaging studies. However, the extent of structural, functional, and metabolic changes in hyperthyroid patients is still unclear. We examined the regional hippocampal volume and neuro-metabolite changes in hyperthyroid patients using high resolution T1-weighed imaging and 1H MRS techniques. Hyperthyroid patients showed reduced bilateral hippocampal volume and altered metabolic changes, i.e., Glu/tCr, mI/tCr, and NAA/tCr ratios in the hippocampus, compared to healthy controls. These findings provide evidence that hyperthyroidism results in structural and metabolic alterations in the hippocampus region of the brain.
Introduction
Hyperthyroidism has been associated with a variety of cognitive and emotional impairments, which include deficits in attention, concentration, memory and depressed mood as suggested by several neuropsychological tests1. The neurocognitive functions such as learning and memory are associated with the hippocampus, which is a core structure of the limbic system and highly sensitive to thyroid hormone concentration2,3. Hyperthyroid patients show reduced grey matter and altered functional connectivity in the hippocampus as evaluated by Voxel based Morphometry (VBM) and resting state functional magnetic resonance imaging (rsfMRI) studies4,5. These studies indicate that thyroid hormones greatly influence metabolism, structure, and function of the brain, in particular the hippocampus. However, the extent of structural, functional, and metabolic changes in hyperthyroid patients is still unclear. Thus, we aimed here to examine the regional hippocampal volume and neuro-metabolite changes in hyperthyroid patients compared to control subjects using high resolution T1-weighted images and proton magnetic resonance spectroscopy (1H MRS). We hypothesized that hyperthyroid patients would show altered hippocampal volumes and neuro-metabolites compared to control subjects.Materials and Methods
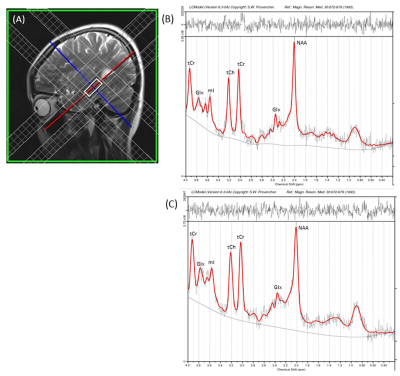
Fourteen hyperthyroid patients (age, 36.0 ± 7.0 years; 5 male) and 14 control subjects (age, 32.1 ± 7.2 years; 7 male) participated in this study. Control subjects were healthy, without any history of chronic medical or psychiatric conditions or head injury, and were recruited from the local area. All the patients were diagnosed with hyperthyroidism for the first time, and were recruited from the Thyroid Research Centre of our Institute. All procedures were approved by the Institutional Review Boards and each subject provided written informed consent prior to the study. Brain MRI scans were acquired using a 3.0 Tesla MRI scanner (Magnetom Skyra, Siemens, Erlangen, Germany). High-resolution T1-weighted images were acquired using a magnetization-prepared rapid gradient echo sequence (MPRAGE) sequence (TR = 1900 ms; TE = 2.07 ms; inversion time = 900 ms; matrix size = 256×256; field of view (FOV) = 256×256 mm2; slice thickness = 1 mm; number of slices = 160). T2-weighted images were collected using a spin-echo pulse sequence in the axial plane (TR = 5600ms, TE = 100ms, number of excitations (NEX) = 2, matrix size=312×512, FOV = 220 mm, 25 slices, slice thickness = 4.0 mm, distance factor = 1.2 mm). The MRS data was acquired using Single Volume Point Resolved Spectroscopy sequence (PRESS) with acquisition parameters: TR/TE = 2000ms/35ms; 2048 spectral points; 1200 Hz spectral Bandwidth and 196 averages. For the hippocampus, axial obliques were obtained by selecting slices parallel to the body of the hippocampus in a parasagittal section, whereas coronal obliques were obtained by positioning slices perpendicular to the body of the hippocampus. A voxel of 25x10x10 mm3 was then positioned on these axial oblique, coronal oblique and sagittal sections for better delineation and coverage of hippocampus. Representative voxel placement in the sagittal plane of a control subject is shown in Figure 1(A). Regional hippocampal volumes were calculated using Freesurfer software (v 6.0.0), and he MRS raw data was processed using LCModel Version 6.3 for quantitative assessment of the brain metabolites. Regional hippocampal volumes and metabolite ratios were examined for significant differences between hyperthyroid patients and controls using ANCOVA, with age and sex included as covariates. We considered a p < 0.05 value statistically significant.Results
No significant differences in age (p = 0.15) or sex (p = 0.46) appeared between groups. Both the left and right hippocampus showed reduced volume in hyperthyroid patients compared to healthy controls ((left, 3610.7±402.7 vs. 4089.2 ± 444.5 mm3, p = 0.001; right, 3786.8±426.2 vs. 4354.4 ± 457.6 mm3, p = 0.001). MRS results revealed a significantly decreased glutamate/creatine (Glu/tCr) (p=0.001), myo-inositol/creatine (mI/tCr) (p=0.048) and N-acetyl aspartate/creatine (NAA/tCr) (p=0.013) ratios in the hippocampus in hyperthyroid patients compared to controls. Hyperthyroid patients showed a significant positive correlation between TSH and NAA/tCr (r = 0.641; p = 0.014). The representative spectra acquired from the right hippocampus of a control and hyperthyroid subject are shown in Figure 1(B and C).Discussion and Conclusion
Hyperthyroid patients showed reduced volume and altered metabolic changes in the hippocampus, which is sensitive to the action of thyroid hormones due to its high content of thyroid receptors3. The reduced Glu/tCr, mI/tCr, and NAA/tCr ratios in hyperthyroid patients possibly indicate alterations in glutamate/glutamine cycle, astrocytic physiology, and/or neuronal loss in the adult human brain. The decreased hippocampal volumes in hyperthyroid patients are in line with the grey matter reduction in the hippocampus as suggested by previous neuroimaging studies4. These findings indicate that hyperthyroidism results in altered structural and metabolic changes in the hippocampus.Acknowledgements
This work was supported by Defence Research & Development Organization (DRDO) R&D Project No. INM 311 (4.1).References
1. Yudiarto FL, Muliadi L, Moeljanto D, et al. Acta medica Indonesiana.2006;38:6-10.
2. Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Nature medicine. 1998; 4:1313-7.
3. Marti-Carbonell MA, Garau A, Sala-Roca J, et al. Acta neurobiologiae experimentalis. 2012;72:230-9.
4. Zhang W SL, Yin X, Zhang J, et.al. European journal of radiology. 2014; 8343-8.
5. Zhang W, Liu X, Zhang Y, et al. European journal of radiology. 2014; 83:1907-13.